Patents
Literature
441results about How to "Reduce production loss" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Heating device for improving heating quality of steel billets and using method thereof
ActiveCN102242249AImprove heating uniformityImprove uneven flow fieldFurnace typesHeat treatment furnacesHeat pipe heat exchangerGas pipeline
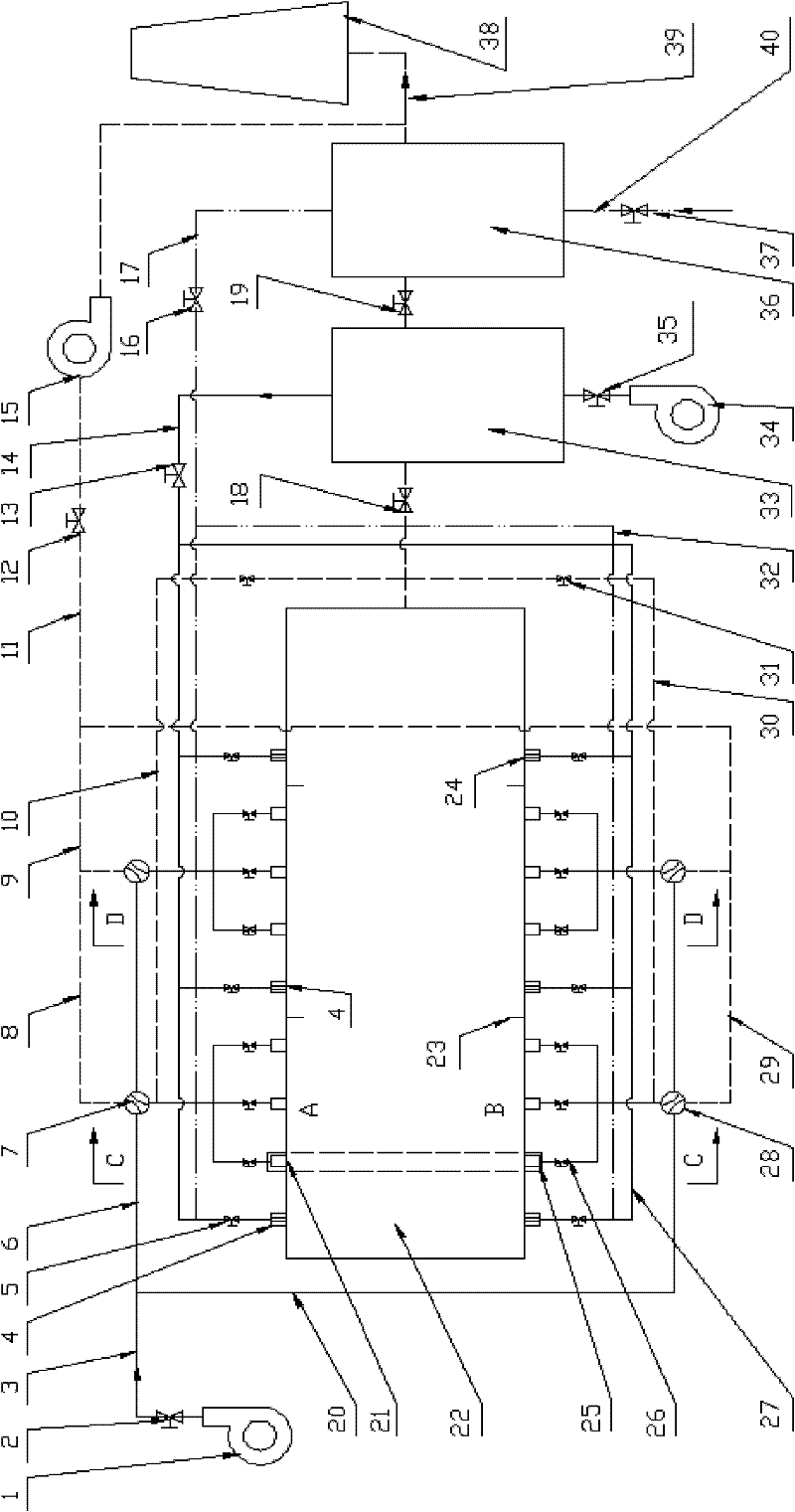
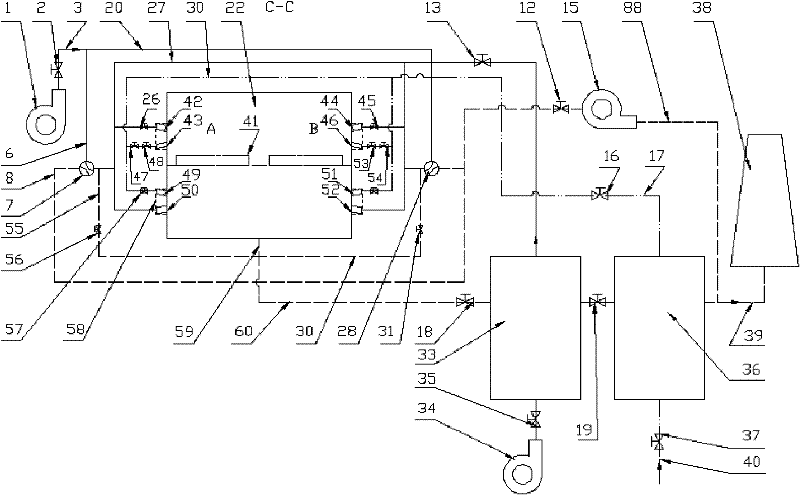
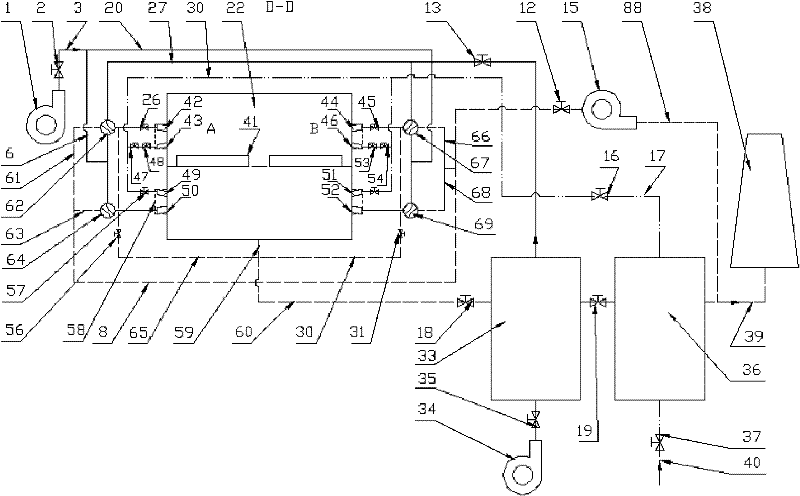
The invention relates to a heating device for improving heating quality of steel billets and a using method thereof. The heating device comprises a heating furnace, a heat storage system blower, a flat flame system blower, a combustion-supporting air pipeline, a gas pipeline, a heat storage system draught fan, an air heat exchanger, a gas heat pipe exchanger, a smoke exhaust pipeline and a chimney. 2-4 ignition burners are arranged at a preheating section of a heating furnace, and 12-30 pairs of heat storage type burners and 4-12 flat flame burners are respectively arranged at a heating section and a soaking zone. The using method comprises the following steps of reasonable distribution of combustion-supporting air and gas flow rate of the heat storage type burners and the flat flame burners, sectional configuration of furnace temperature and heating time, preheating of combustion-supporting air and gas temperature through a high-temperature smoke gas and furnace tail gas in grades, alternative furnace feeding of cold and heat steel billets, intermittent movement of the steel billets, and vaporization cooling of the steel billets. The flow field in the furnace is modified through the system, the optimal configuration of flow rates of air and gas in the burner is realized through reasonable arrangement of gas and air pipeline systems, and the waste heat of high-temperature smoke gas of the heating furnace is gradiently utilized, thus the in-furnace time is shortened, the uniformity of heating the steel billets is improved, and the high-efficiency heating of the steel billets is realized.
Owner:SHOUGANG CORPORATION
Method for processing furnace roller nodules on line without opening furnace
ActiveCN105385836AEasy to useExtend economic lifeFurnace typesHeat treatment furnacesEngineeringStrip steel
The invention discloses a method for processing furnace roller nodules on line without opening a furnace. Due to the fact that the operating speed of band steel in the furnace is the same as the linear speed of furnace rollers, the diameter of the furnace rollers is corrected, and accordingly a new angular speed is produced by a furnace roller motor so as to be the same as the linear speed of other furnace rollers. According to the control scheme, the diameter of the furnace rollers to be repaired and ground is adjusted analogously by an operator so as to change the speed of the furnace rollers, and accordingly friction is generated between the furnace roller and the band steel, so that the nodules attached to the surfaces of the furnace rollers are eliminated or smoothed. Compared with the prior art, by means of the method for the furnace roller nodules in the initial stage, the quality deflects such as hard spots on the surfaces of the band steel are effectively relieved or eliminated, the surface quality of a finished roll is improved, the frequency of furnace opening to clean the furnace roller nodules annually is decreased, and the service life and the economic life of the furnace rollers are prolonged.
Owner:MAANSHAN IRON & STEEL CO LTD
Energy-saving cluster control system and method of air compression station house
ActiveCN104820413ARealize automatic operationReduce operating costsProgramme total factory controlCooling towerEngineering
The invention relates to an energy-saving cluster control system and method of an air compression station house. The system comprises an air pipe pressure sensor used for acquiring pressure of compressed air; an air pipe flowmeter used for acquiring the flow of the compressed air; a dew point instrument used for acquiring dew points of the compressed air; a water pipe pressure sensor used for acquiring the outlet water pressure of circulating water pumps; a cooling tower temperature sensor used for acquiring the outlet and inlet water temperatures of cooling towers; an inflator central controller connected with inflators through a CAN bus; and a PLC respectively connected with dryers, the circulating water pump, cooling blowers, electric valves, the air pipe pressure sensor, the air pipe flowmeter, the dew point instrument, the water pipe pressure sensor, the cooling tower temperature sensor and the inflator central controller. Compared to the prior art, the energy-saving cluster control system has the advantages of saved energy consumption, accurate control, convenient operation and maintenance and the like.
Owner:NINGBO CITY HANGZHOUWAN NEW DISTRICT XIANGYUAN POWER SUPPLYING
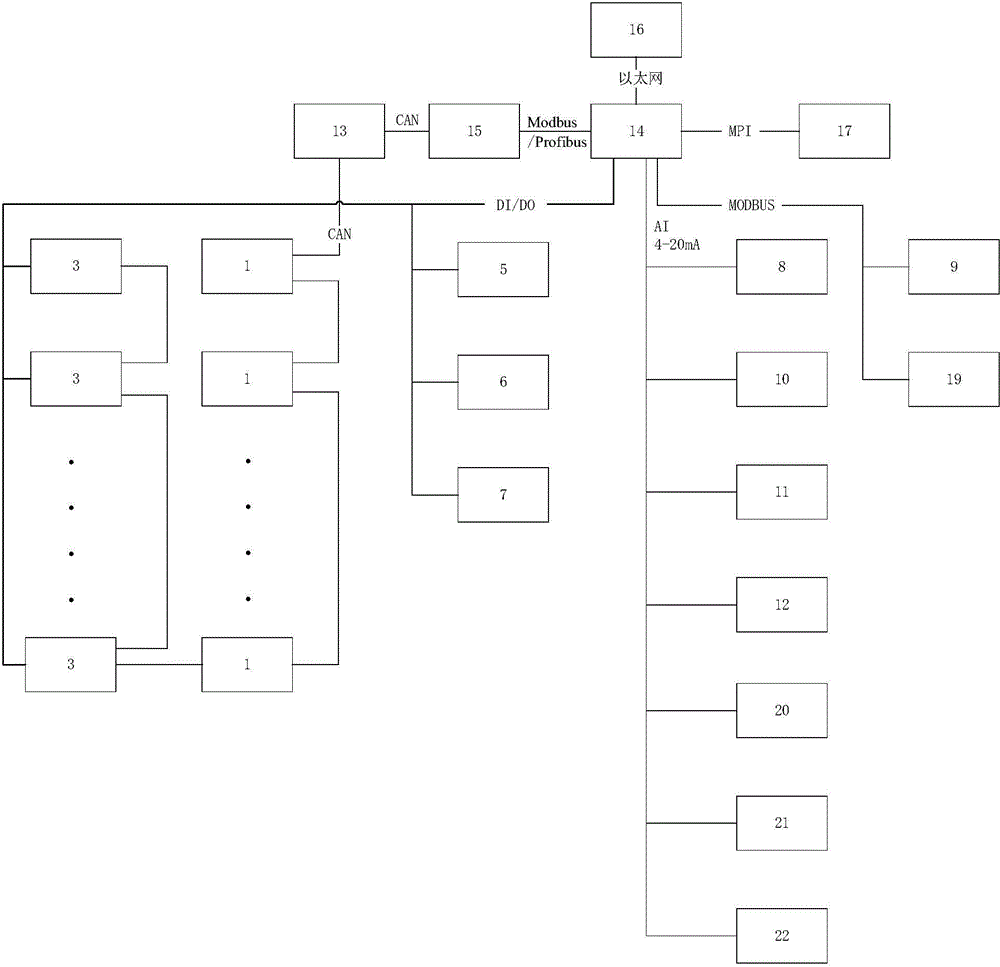
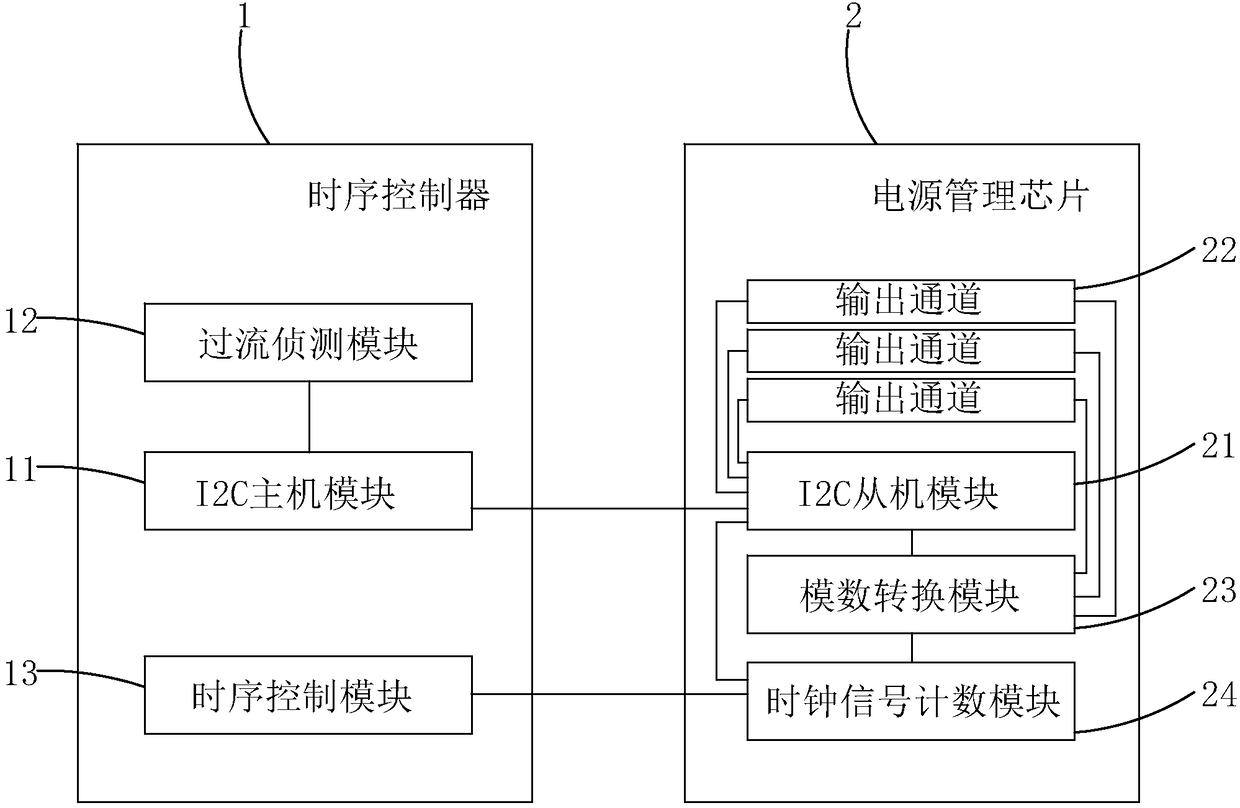
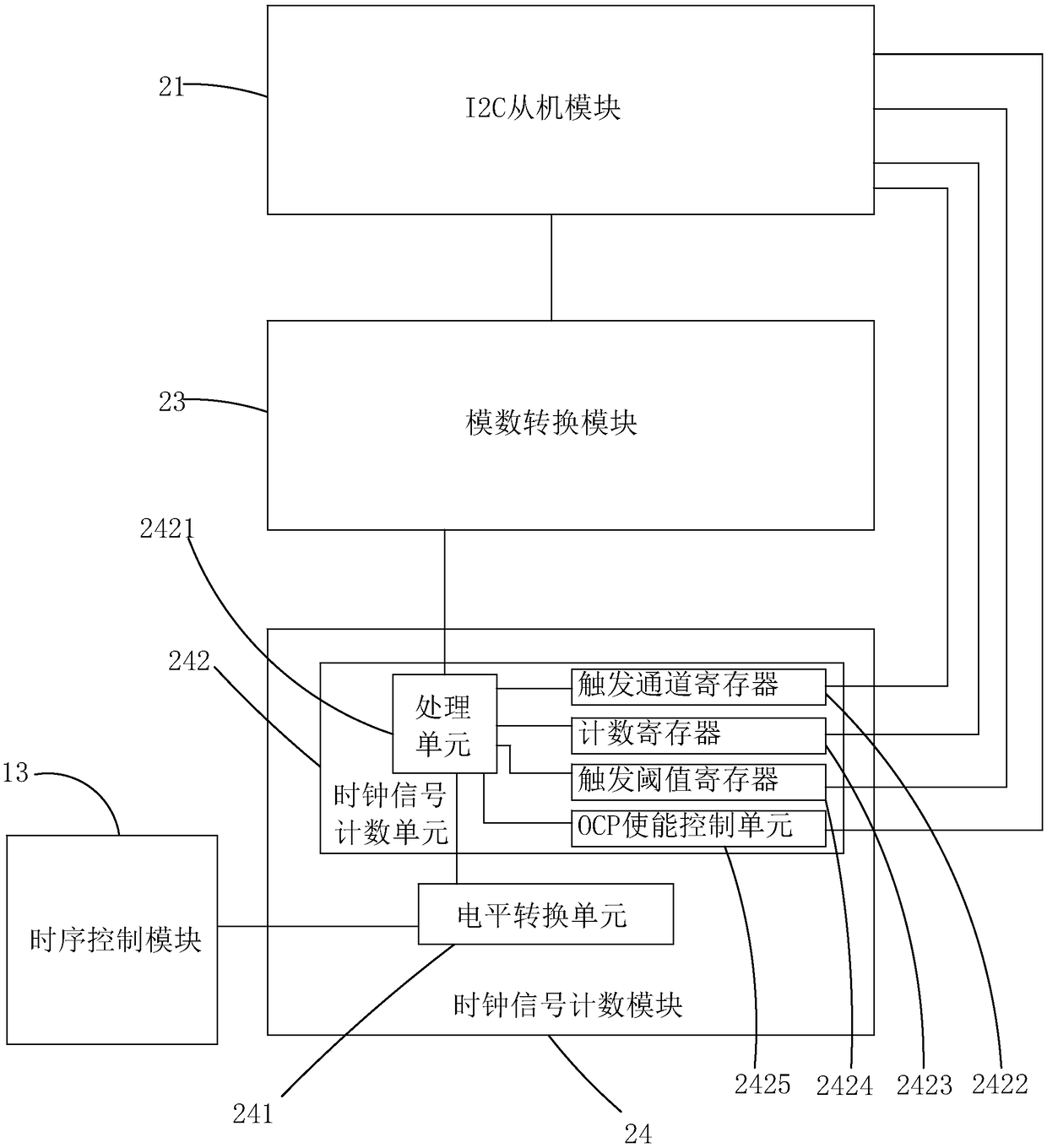
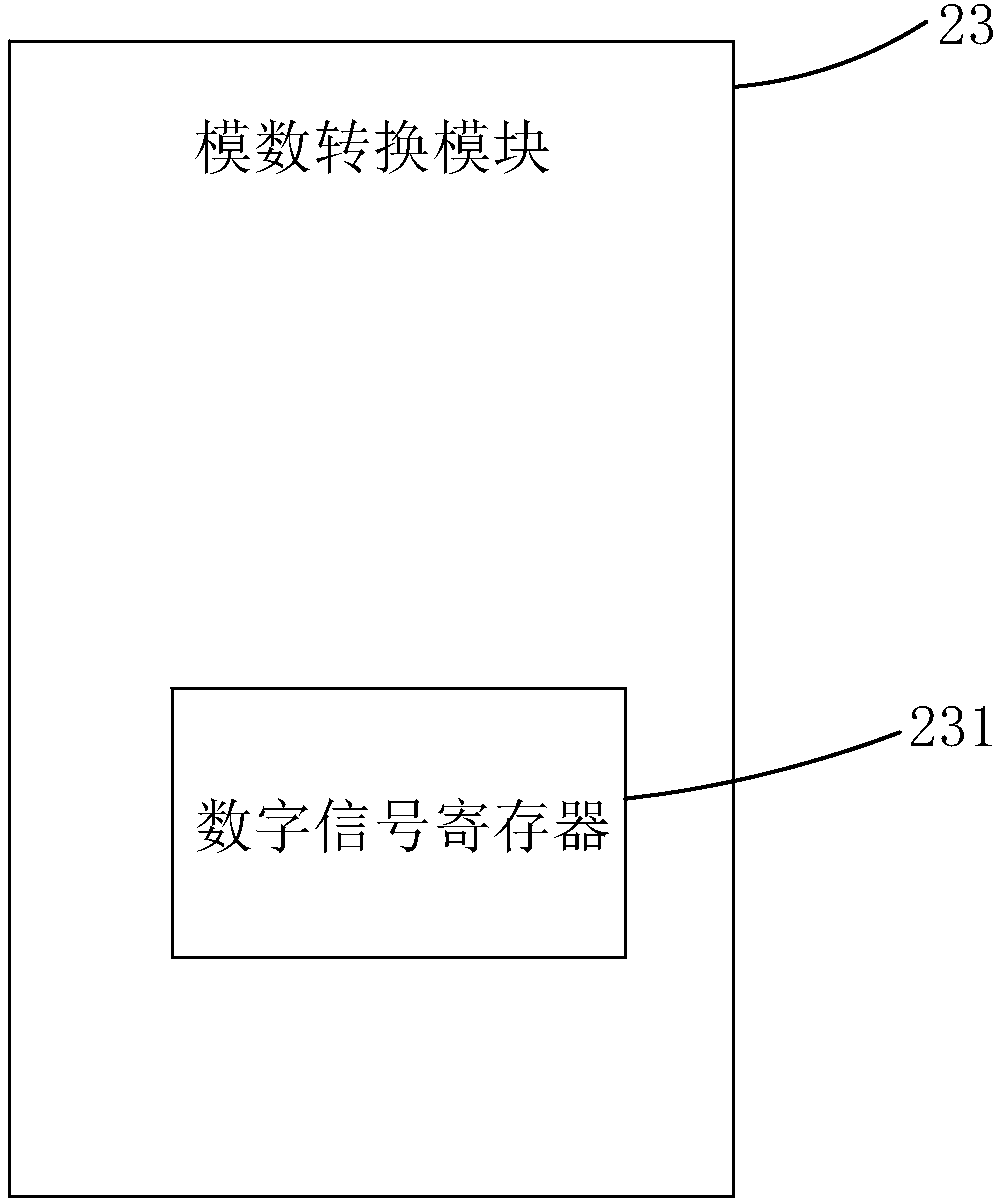
Over-current protection system and over-current protection method for liquid crystal display panel
ActiveCN108550350AImprove the internal circuitAvoid overcurrent burningStatic indicating devicesArrangements responsive to excess currentDriving currentLiquid-crystal display
The invention provides an over-current protection system and over-current protection method for a liquid crystal display panel. The over-current protection system is composed of a time sequence controller and a power management chip. The time sequence controller consists of an I2C host module, an over-current detection module and a time sequence control module. The power management chip includes an I2C slave module, a plurality of output channels, an analog-to-digital conversion module and a clock signal counting module. The I2C host module sends an output channel selection instruction; the clock signal counting module sends out a collection instruction based on a starting signal outputted by the time sequence control module and a plurality of clock signals; after the analog-to-digital conversion module receives the collection instruction, a driving current outputted by the corresponding output channel is collected based on the output channel selection instruction and a digital signalis obtained; and the over-current detection module determines whether the driving current is in an over-current state based on the digital signal. Therefore, over-current detection accuracy is improved and the internal circuitry of the liquid crystal display panel is protected.
Owner:SHENZHEN CHINA STAR OPTOELECTRONICS TECH CO LTD
Polyvinyl acetal film and uses thereof
ActiveUS20130074910A1Reduce yellownessGood lookingPV power plantsGlass/slag layered productsPolyvinyl alcoholOxygen
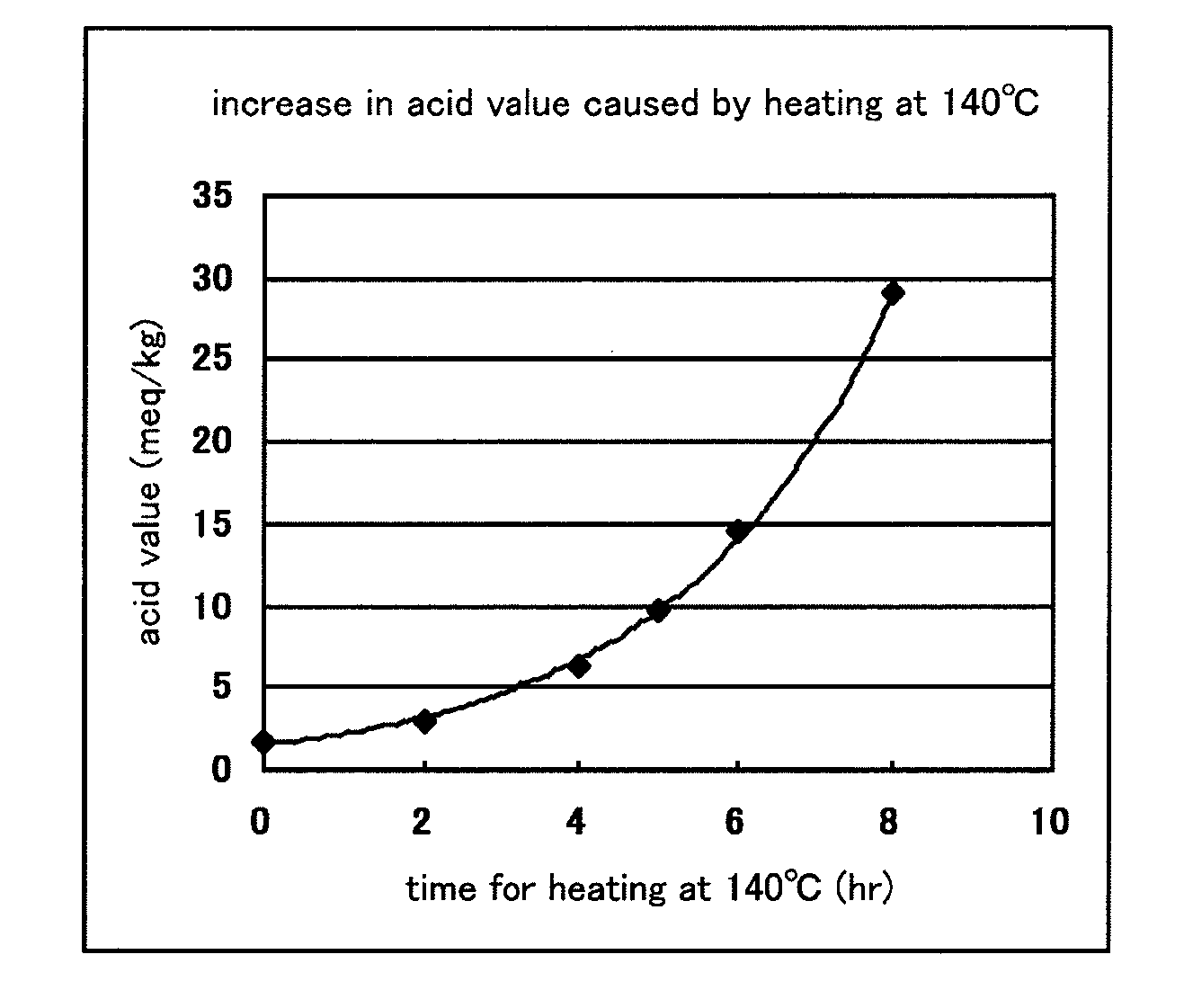
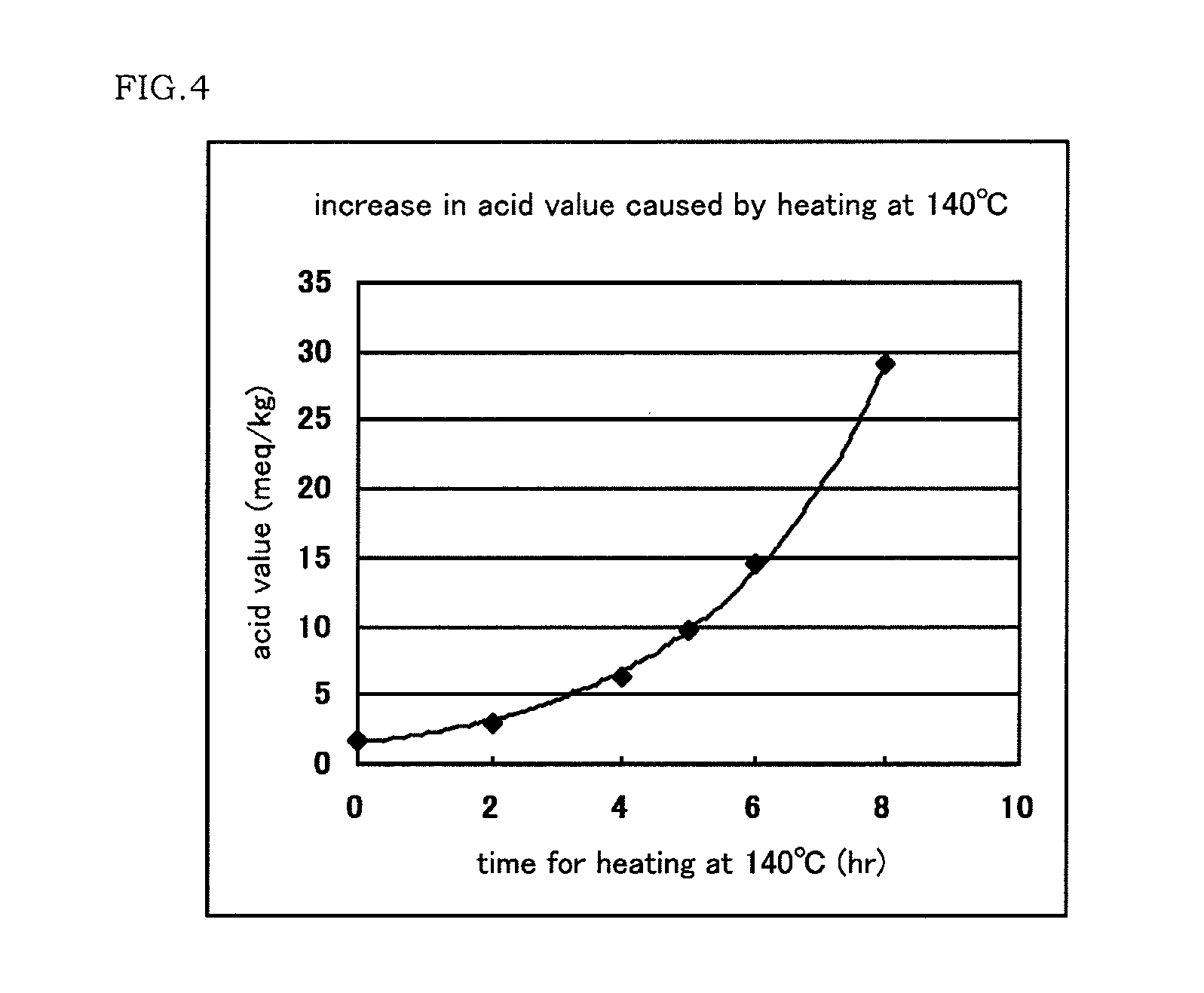
A polyvinyl acetal film as an intermediate film for a laminated glass, can provide a laminated glass that exhibits a low degree of yellowness and excellent surface appearance, and is useful as a sealing material or intermediate film that can prolong the life of a laminated glass provided with a solar cell or functional unit. The content of corrosion-causing substance in the polyvinyl acetal film is low, so that the polyvinyl acetal film permits high-temperature lamination and ensures excellent productivity. A solar cell module and a laminated glass are prepared using the polyvinyl acetal film. A plasticized polyvinyl acetal film which comprises 15 to 60 parts by mass of a plasticizer having a total number of 28 or more of carbon atoms and oxygen atoms constituting a molecule based on 100 parts by mass of a polyvinyl acetal resin, and which has an acid value of 5.0 meq / kg or less.
Owner:KURARAY CO LTD
Method for preparing dehydrated starch products
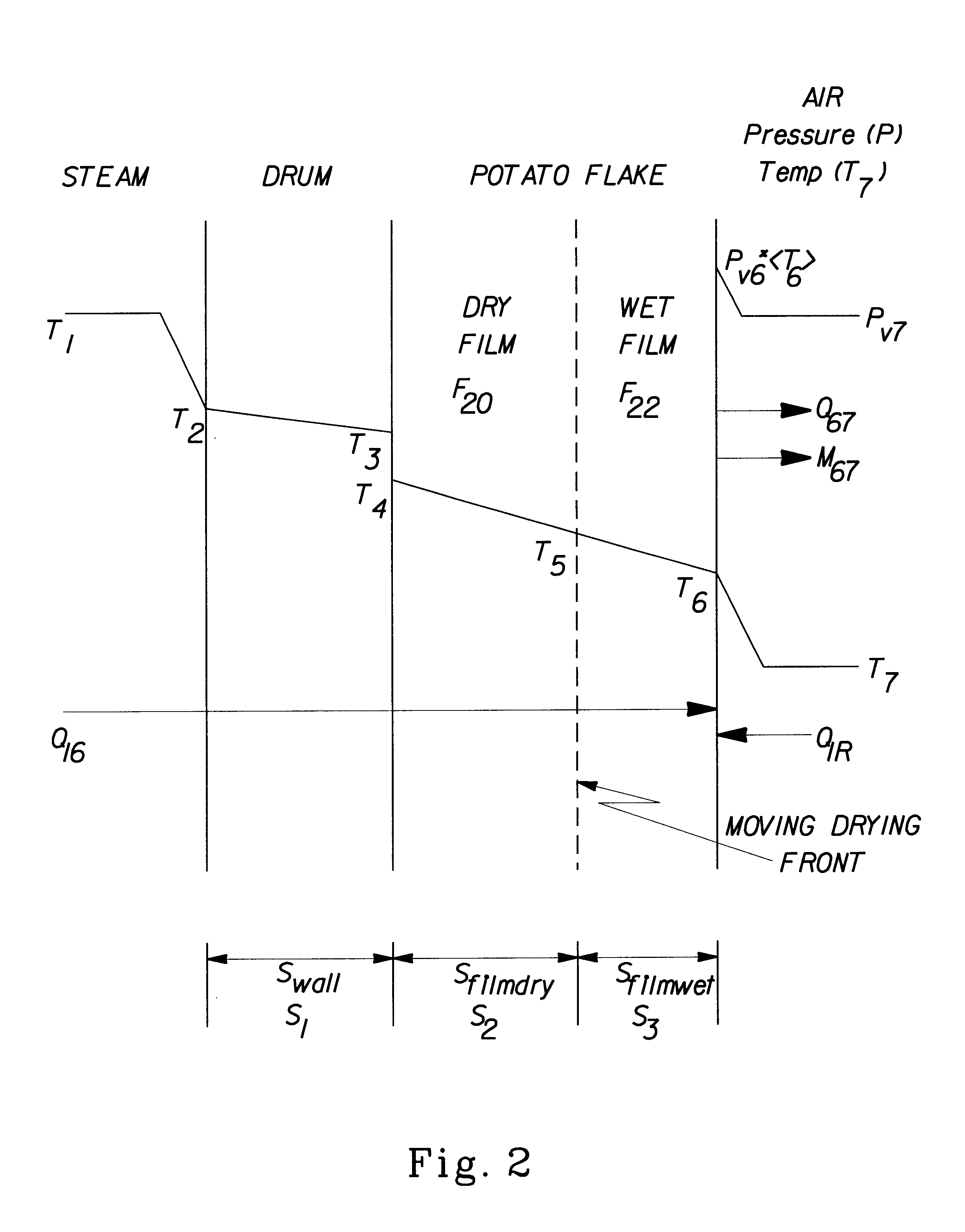
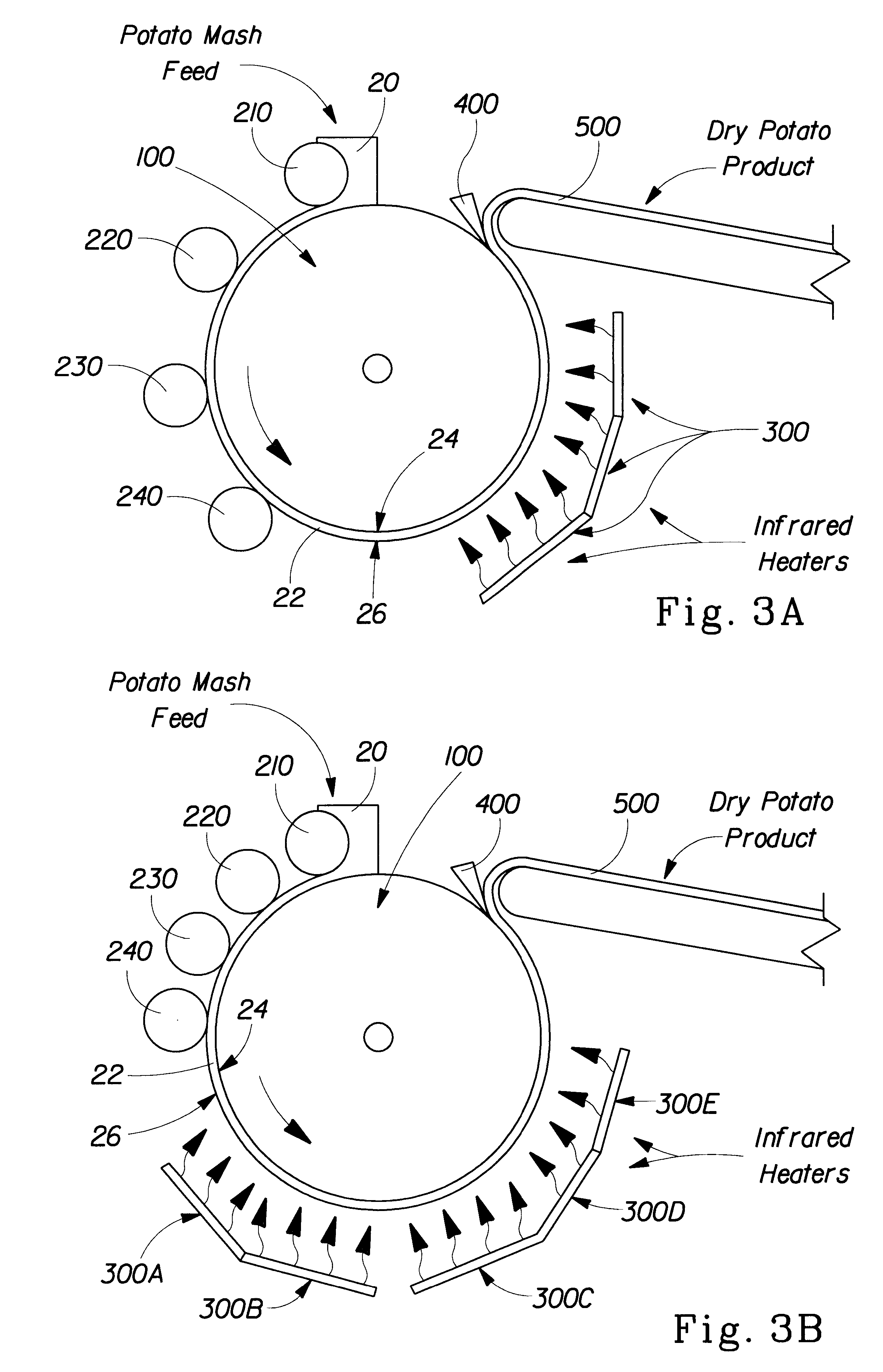
InactiveUS6890580B1Reduce production lossIncrease productionFood preparationFruits/vegetable preservation by dehydrationPlant TubersChemistry
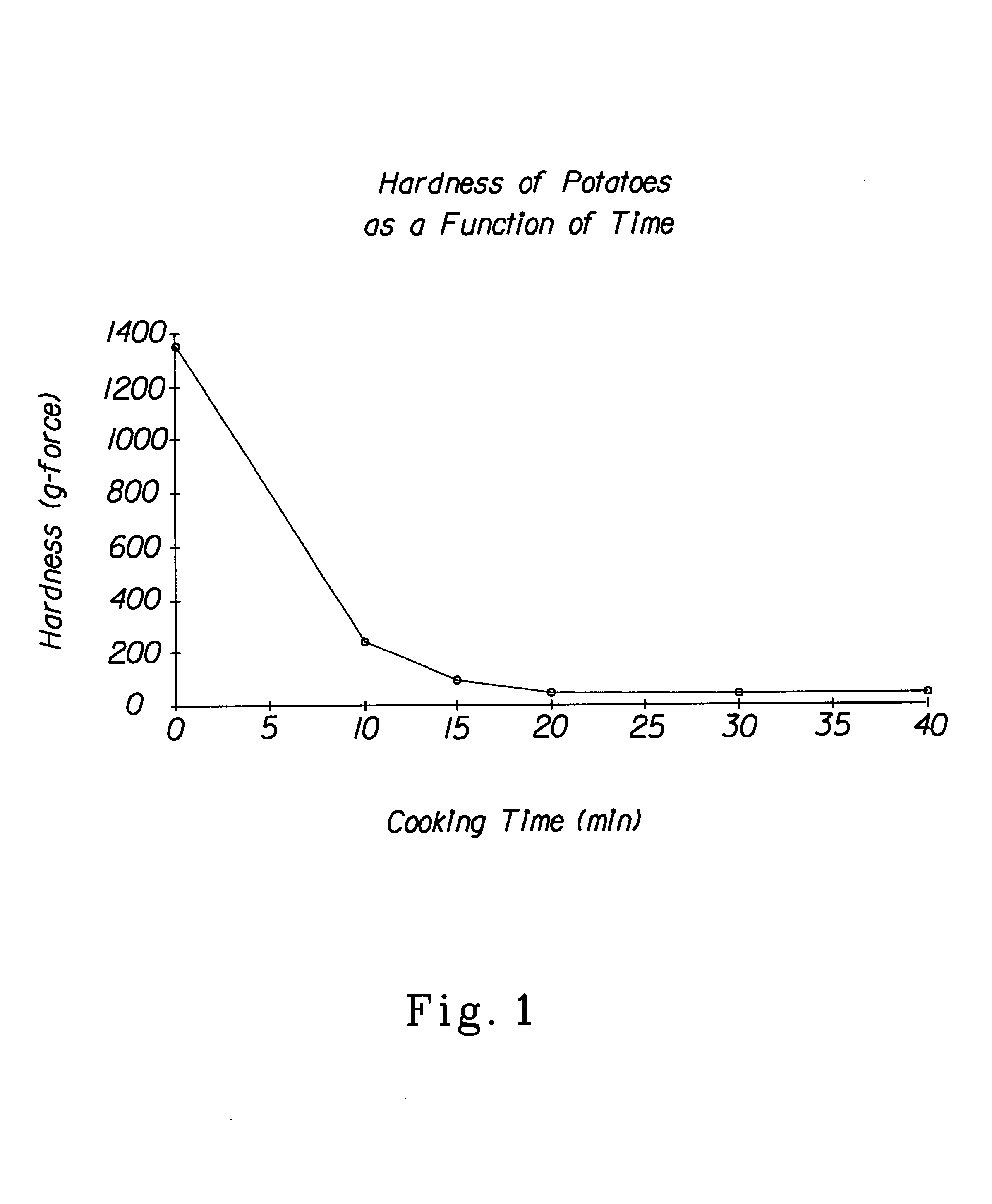
A method for preparing dehydrated fruits, vegetables and tubers, particularly potato products. The steps of the method include: boiling the whole raw edible product or slices or pieces of the raw edible product for a time sufficient to partially cook the starch and soften the tissue of the edible product; comminuting the cooked product; and drying the comminuted product. The raw edible product is preferably unpeeled or partially peeled. The resulting dehydrated product: (1) retains more Vitamin C; (2) has a lighter color; (3) has a higher percent of free amylose; (4) has fewer broken cells; and (5) has fewer degradation products than a corresponding product produced by conventional methods.
Owner:KELLOGG NORTH AMERICA
Chemical industry production site patrol system based on internet of things technology
InactiveCN103714434AChange uncontrollableImprove production management levelChecking time patrolsTechnology managementChemical industryThe Internet
The invention discloses a mechanical industry production site patrol system based on the internet of things technology. An MVC mode is adopted for the system. The system comprises a presentation layer, a service logic layer and a database layer, wherein the presentation layer is used for presenting functions of the system and interacting with a user, and the presentation layer of the system includes two forms, namely, a user browser end and a handheld patrol instrument. The service logic layer is used for data exchange between the database layer and the presentation layer, dispatching requests of the user, selecting an appropriate presentation method for display, explaining input of the user, and mapping the input of the user to the executable operation of the database layer. The database layer is composed of all levels of database resources.
Owner:SHANGHAI HUAYI INFORMATION TECH
Liquid composition capable of removing sulfide in gas
InactiveCN101507891AGood effectEfficient removalDispersed particle separationCombustible gas purificationThiazoleFatty amine
The invention provides a liquid composition for removing sulfide in gas, which consists of an absorbent, an auxiliary agent and an antifoaming agent, wherein the absorbent consists of steric hindrance amine and alkanolamine; the auxiliary agent is one or a mixture of thiazoles, fatty amine and phenols; and the antifoaming agent is siloxane. The liquid composition comprises the following components in percentage by weight: 29 to 89 percent of absorbent, 10 to 70 percent of auxiliary agent and 0.01 to 1 percent of antifoaming agent. The liquid composition can simultaneously and effectively remove organic sulfur and inorganic sulfur in the gas, has obvious desulfurization effect, can effectively protect production equipment, and reduces the loss and the maintenance cost.
Owner:江苏大海能源科技有限公司
Device and method for cleaning tundish upper water gap nodules
ActiveCN108176844AImprove continuous casting outputReduce production lossMelt-holding vesselsEngineeringSteel bar
The invention belongs to the technical field of steel metallurgy and particularly relates to a device and method for cleaning tundish upper water gap nodules. In the continuous casting process, when the tundish upper water gap nodule phenomenon appears, the opening degree of a plug bar is turned down, the pulling speed is reduced to 20-30% of the normal pulling speed, a segment of round steel barcontaining a calcium core is inserted into the tundish upper water gap from the hollow plug bar, steel shells at concave points or concave lines are molten by steel liquid, concave holes or gaps appear in the surface of the periphery of the round steel bar, the calcium core inside the round steel bar is heated to be a gaseous state, the gaseous calcium is sprayed out from the concave holes or thegaps to Al2O3 nodules inside the tundish upper water gap, and the calcium reacts with the Al2O3 nodules. By means of the device and method for cleaning the tundish upper water gap nodules, the cleaning time for the Al2O3 nodules is just 1-5 minutes which is far less than the time for continuous casting order replacement, the improvement of continuous casting production quantity is facilitated, thecaused production loss is far less than that caused by the replacement of a tundish, the plug bar will not be eroded, and the service life of the plug bar will not be shortened.
Owner:LAIWU STEEL YINSHAN SECTION CO LTD
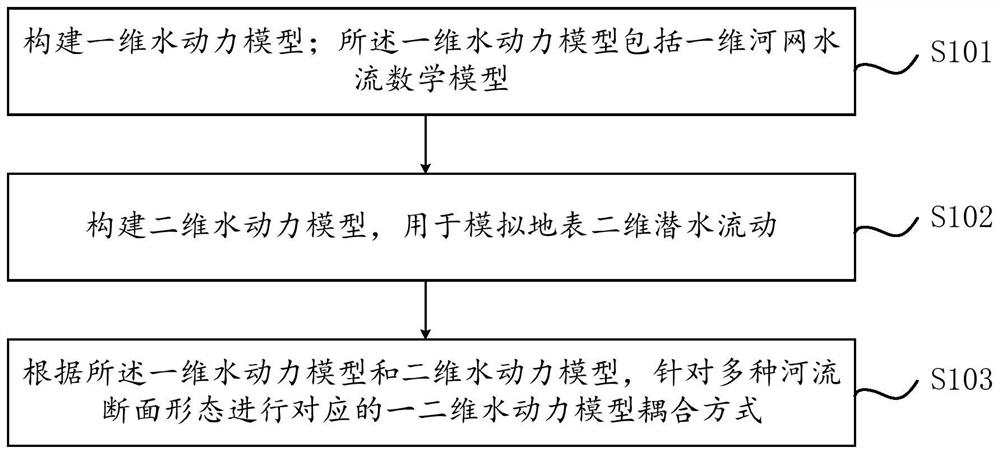
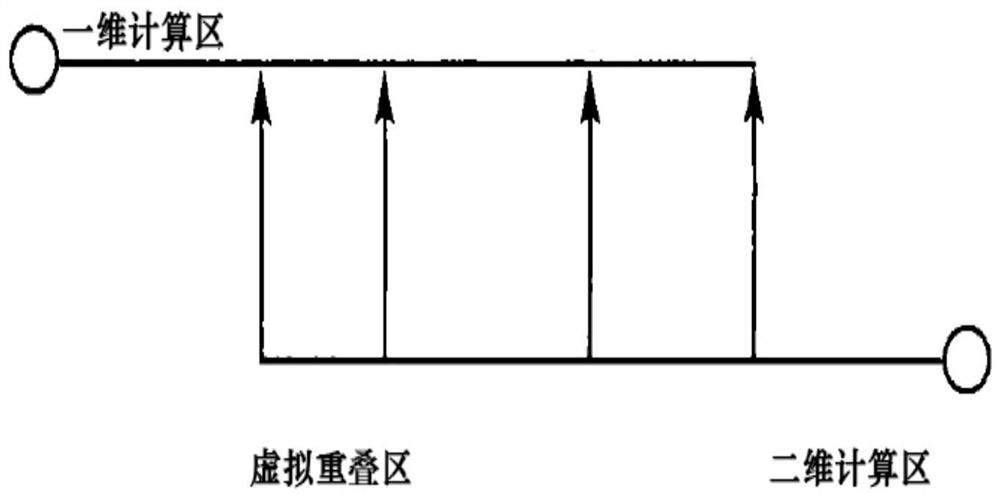
Coupling method and system for one-dimensional hydrodynamic model and two-dimensional hydrodynamic model
PendingCN112257352ARealize resourcesReduce the peak flow of the flood dischargeClimate change adaptationDesign optimisation/simulationRiver networkMathematical model
The invention relates to a coupling method and system for a one-dimensional hydrodynamic model and a two-dimensional hydrodynamic model. The method comprises steps that the one-dimensional hydrodynamic model is constructed; wherein the one-dimensional hydrodynamic model comprises a one-dimensional river network water flow mathematical model; a two-dimensional hydrodynamic model is constructed to simulate two-dimensional shallow water flow of an earth surface; and according to the one-dimensional hydrodynamic model and the two-dimensional hydrodynamic model, a corresponding one-dimensional andtwo-dimensional hydrodynamic model coupling mode is carried out for various river section forms. According to the method, a one-dimensional and two-dimensional coupled hydrodynamic model is adopted, the influence of urban buildings, trees and other plants on flood propagation can be fully considered, and hydrodynamic models of different scales and different dimensions are coupled according to theone-dimensional flow characteristics of a river channel and the two-dimensional flow characteristics of a lake; and flood influence degree and range simulation and prediction under different flood frequencies are carried out. The urban water regime situation can be perceived, the water level information is collected in combination with an upstream sensing instrument, and the aims of reducing the discharge peak flow and achieving flood recycling are achieved in combination with gate dam dispatching.
Owner:YELLOW RIVER INST OF HYDRAULIC RES YELLOW RIVER CONSERVANCY COMMISSION
Method for preparing dapoxetine hydrochloride
ActiveCN106883133AAvoid splittingSolve the serious problem of lossOrganic compound preparationAmino-hyroxy compound preparation3-amino-3-phenylpropionic acidBorohydride
The invention discloses a method for preparing dapoxetine hydrochloride. The method comprises the following steps: subjecting (s)-3-amino-3-phenylpropionic acid or an ester thereof to a reduction reaction in a reduction system prepared from a hydroborate and a boron trifluoride complex, so as to obtain an intermediate 1, i.e. (s)-3-amino-3-phenylpropanol; subjecting the (s) intermediate 1 to an Eschweiler-Clark reaction with formic acid and formaldehyde, so as to obtain an intermediate 2; subjecting the intermediate 2 to a Williamson ether forming reaction with 1-fluoronaphthalene, so as to obtain a free alkali, i.e. (s)-N,N-dimethyl-3-(1-naphthyloxy)phenyl propyl amine; subjecting the free alkali to a salt forming reaction with alcohol-acyl chloride or a chloride thereof, a hydrochloric acid organic solution or hydrochloric acid gas, thereby obtaining dapoxetine hydrochloride. According to the method, the synthesis route is low in production cost, the reaction conditions are mild, all the materials are readily available, the raw materials are low in toxicity, the reaction is simple in operation and high in safety, and the product is high in purity and yield and is environmentally friendly, so that the method is applicable to industrial large-scale production.
Owner:SPRINGPHARMA CO LTD
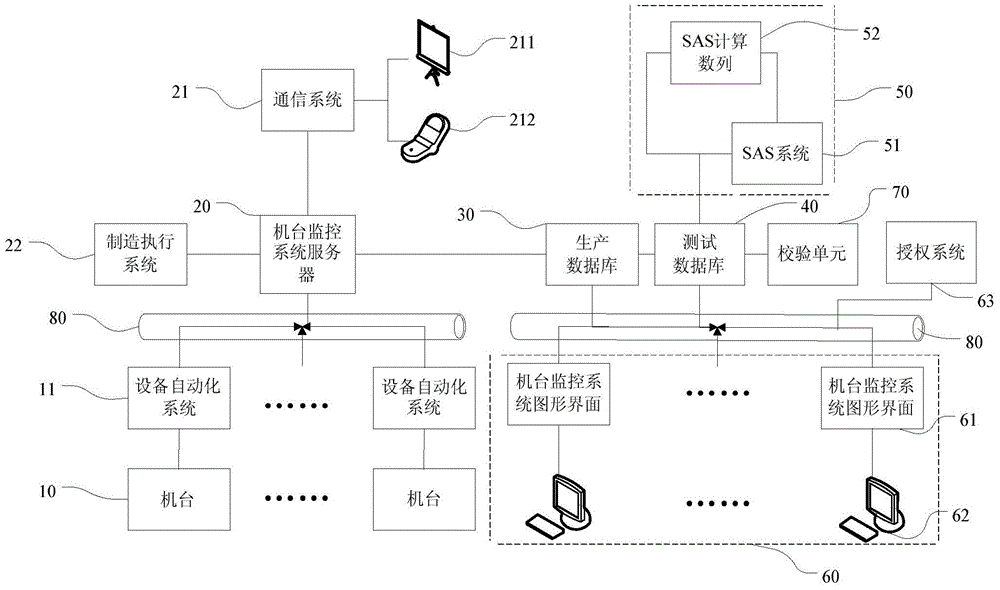
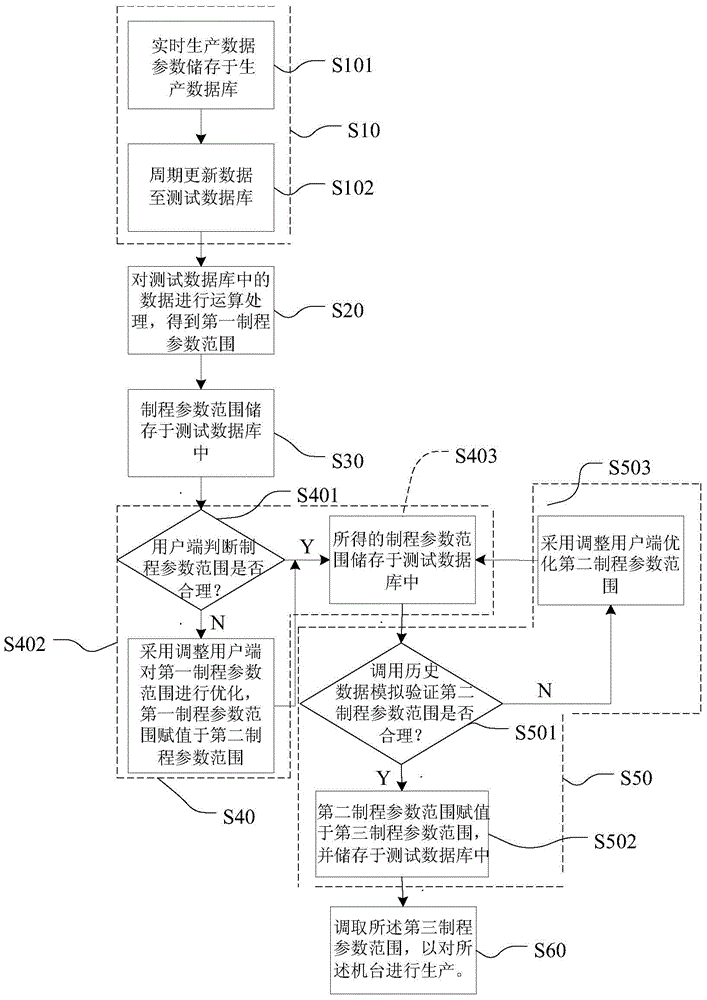
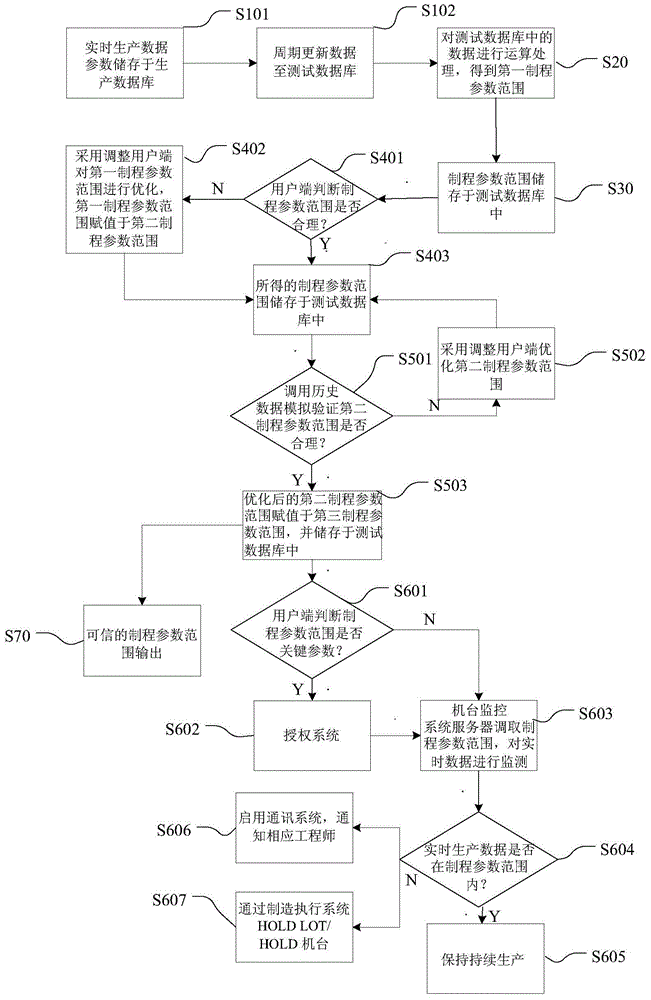
System used for controlling machine production data and method thereof
ActiveCN105223914AHigh feasibilityTake advantage of reliabilityTotal factory controlProgramme total factory controlMonitoring systemForward looking
The invention discloses a system used for controlling machine production data and a method thereof. The system comprises a test database, a data processing unit, a verification unit, a machine monitoring system server and an adjusting client. The method comprises steps that, production data parameters are acquired and are stored in the test database; operation processing on the acquired data is carried out to acquire a process parameter scope; data parameters are adjusted by employing the adjusting client and according to historical data to acquire a required progress parameter scope. Through the system and the method, reference values of all parameters under all conditions in the process scope can be figured out through calculation, determination is carried out according to experience and the calculation results, forward-looking determination for the process parameter scope is realized, error operation is reduced, influence of the error operation on a real-time production system is further reduced, and thereby validity and accuracy of the process parameter scope are improved.
Owner:SEMICON MFG INT (SHANGHAI) CORP
Wear-resistant nylon composite material and preparation method thereof
The invention belongs to the technical field of engineering plastics, and concretely relates to a wear-resistant nylon composite material and a preparation method thereof. The wear-resistant nylon composite material comprises, in parts by weight, 100 parts of nylon, 6-20 parts of a solid lubricant, 30-35 parts of glass fibre, 12-15 parts of teflon, 3-7 parts of silicone powder, 4-10 parts of a compatibilizer, and 1-2 parts of an anti-oxidant, wherein the solid lubricant comprises 40-70 parts of molybdenum disulfide and 30-60 parts of tungsten sulfide, is prepared by uniformly mixing the two compositions and performing ball milling in a ball mill at a rotation speed of 250-350 r / min for 6-12 h, and has the particle size of 300-400 nm.
Owner:CHANGSHA HANWEI DAXIN MSTAR TECH
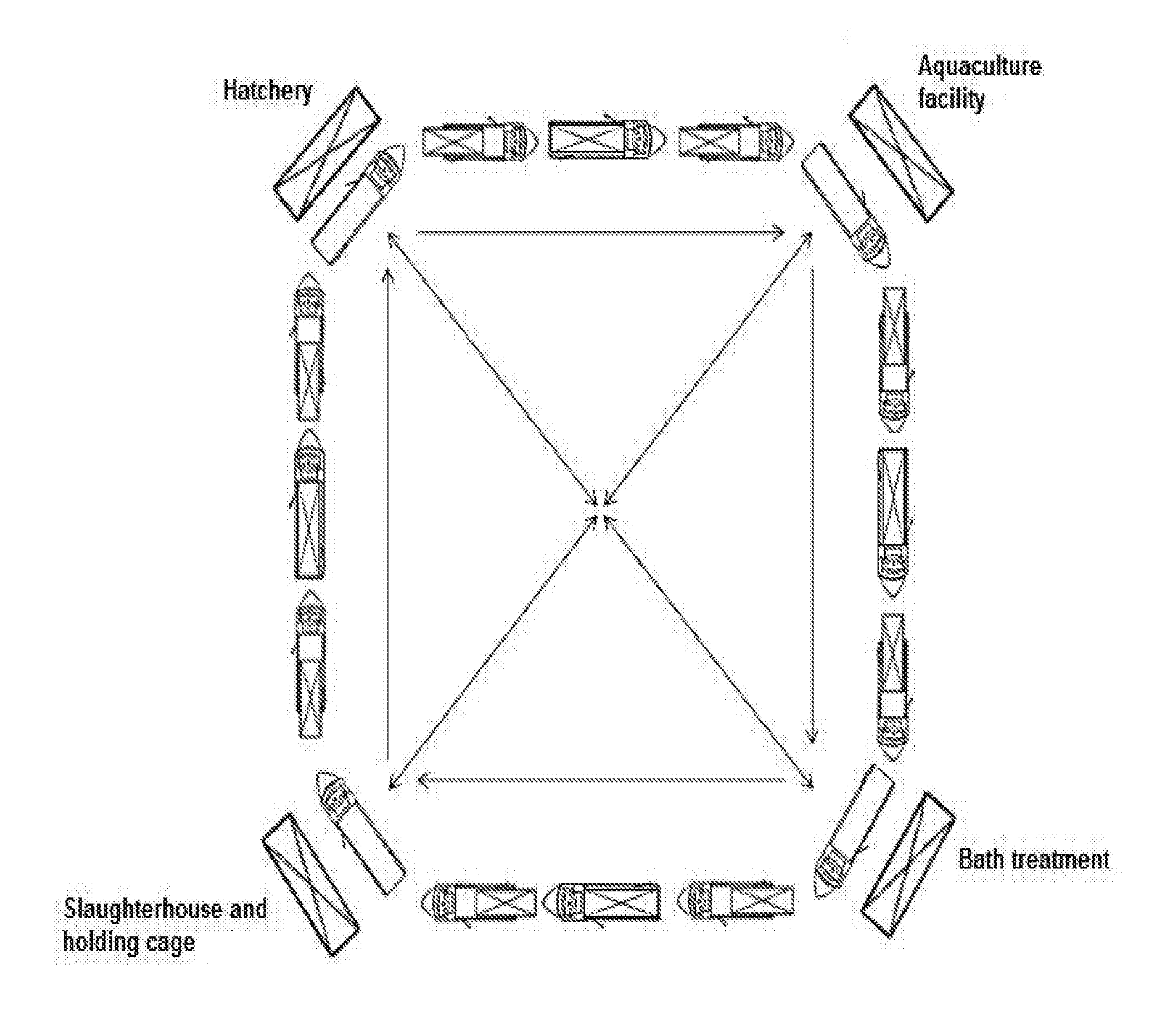
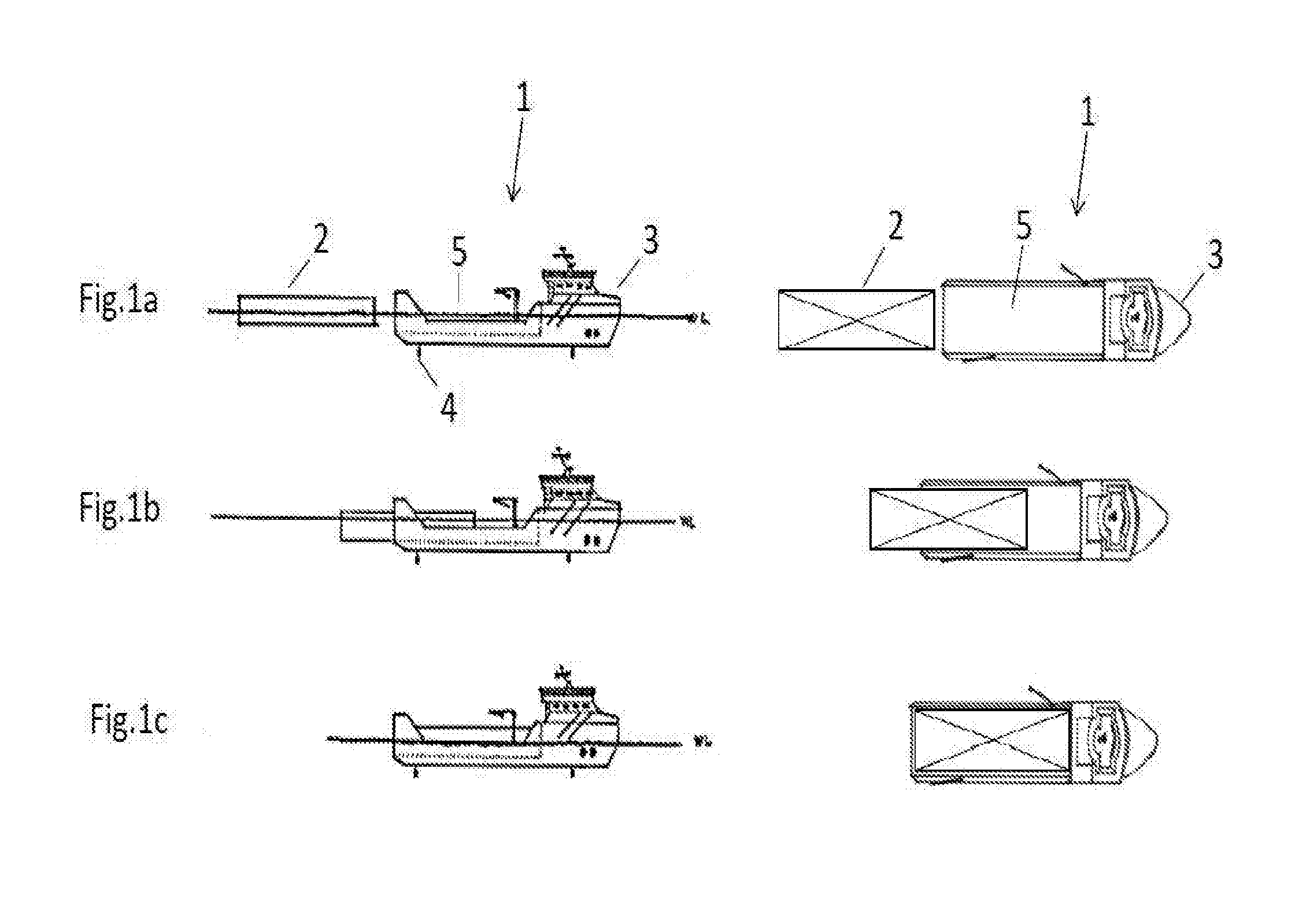
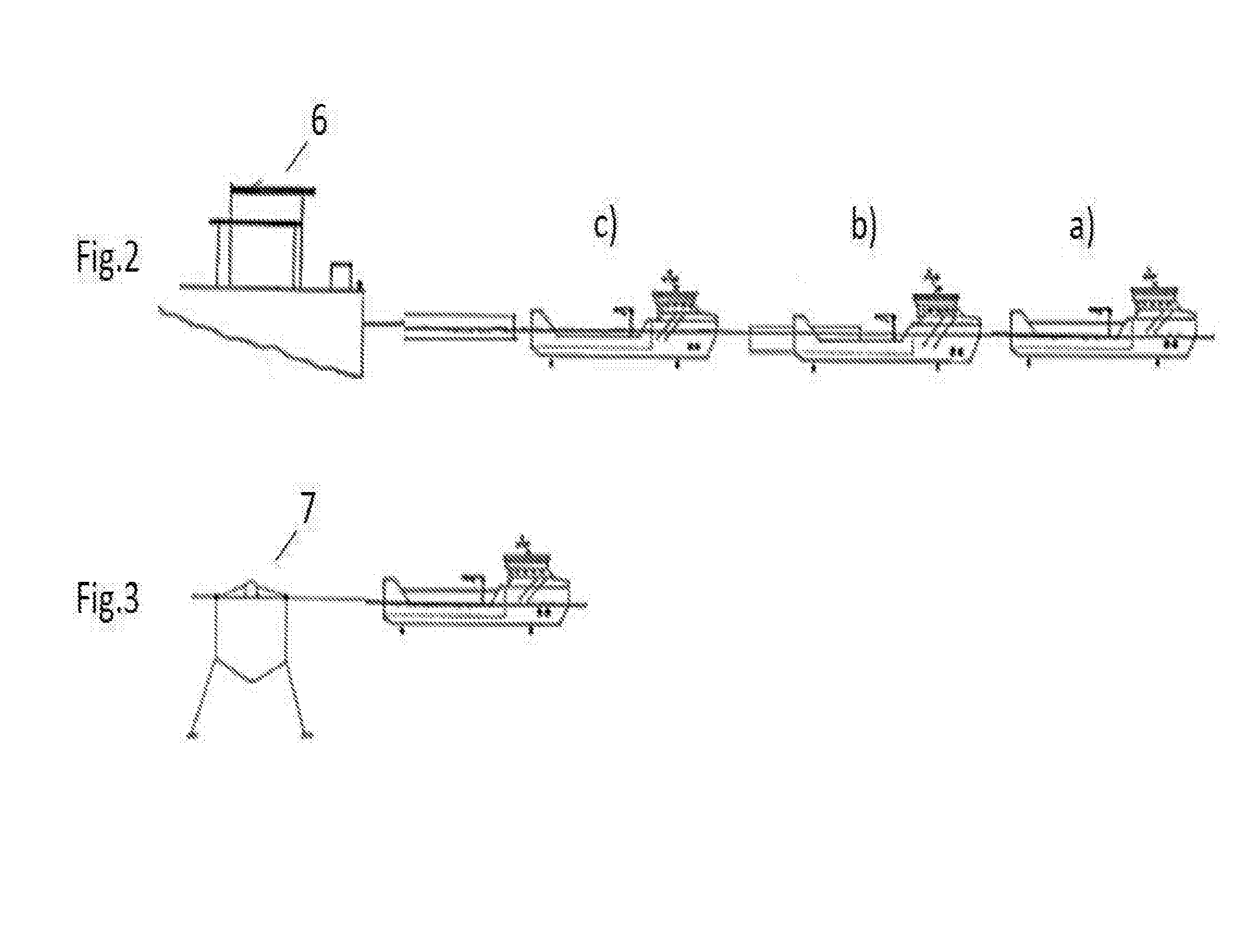
Well and service vessel for transport and storage of aquatic organisms
InactiveUS20160244130A1More cost-effectiveMore time-effectiveCargo handling apparatusFishing netsMarine engineeringOedogonium
An arrangement, a system and a method relating to a well boat- and service vessel (1) for transporting or storing fish or other aquatic organisms. There are provided, inter alia, a well boat- and service vessel (1) that includes a self-floating, exchangeable well unit (2), a propulsion unit (3), wherein the propulsion unit includes propulsion elements (4), steering and positioning elements, a dock (5) for the well unit, positioning and attachment elements for positioning and securing the well unit (2) in the dock (5).
Owner:MOOD FREDRIK
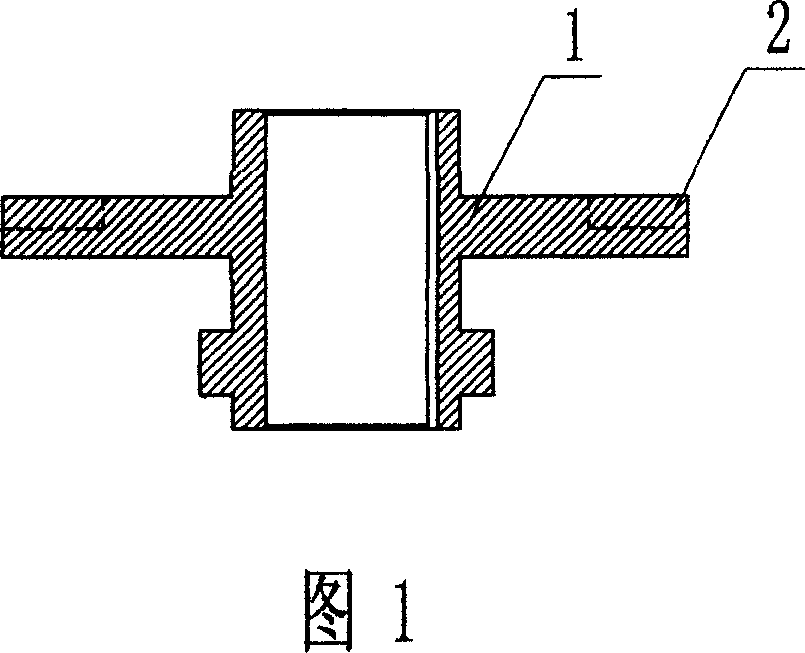
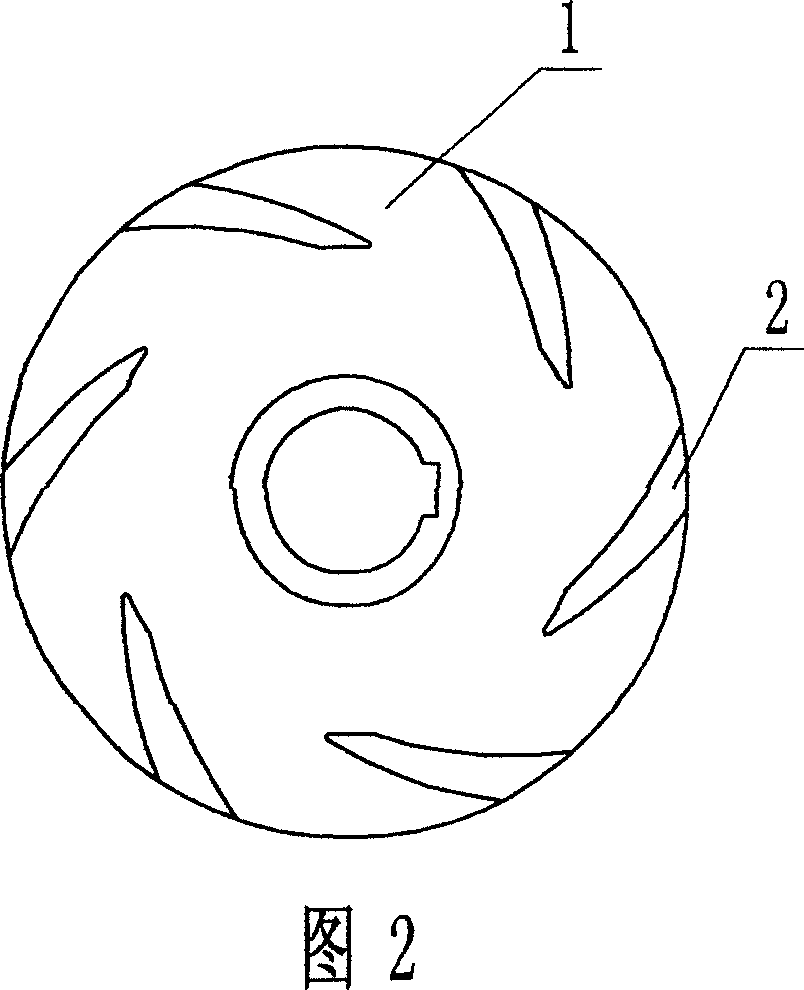
Combined antiwearing blades
InactiveCN1975172AExtended service lifeReduce damage frequencyPump componentsPumpsEngineeringEnergy conservation
Owner:宜兴市宙斯泵业有限公司
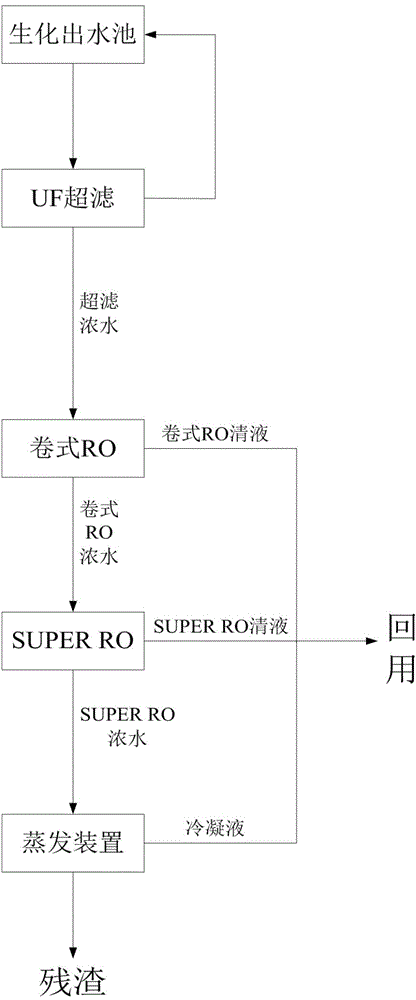
Zero-discharging technology in reclaimed water reuse
ActiveCN104150718AIncrease the concentration factorReduced size and operating costsMultistage water/sewage treatmentUltrafiltrationReclaimed water
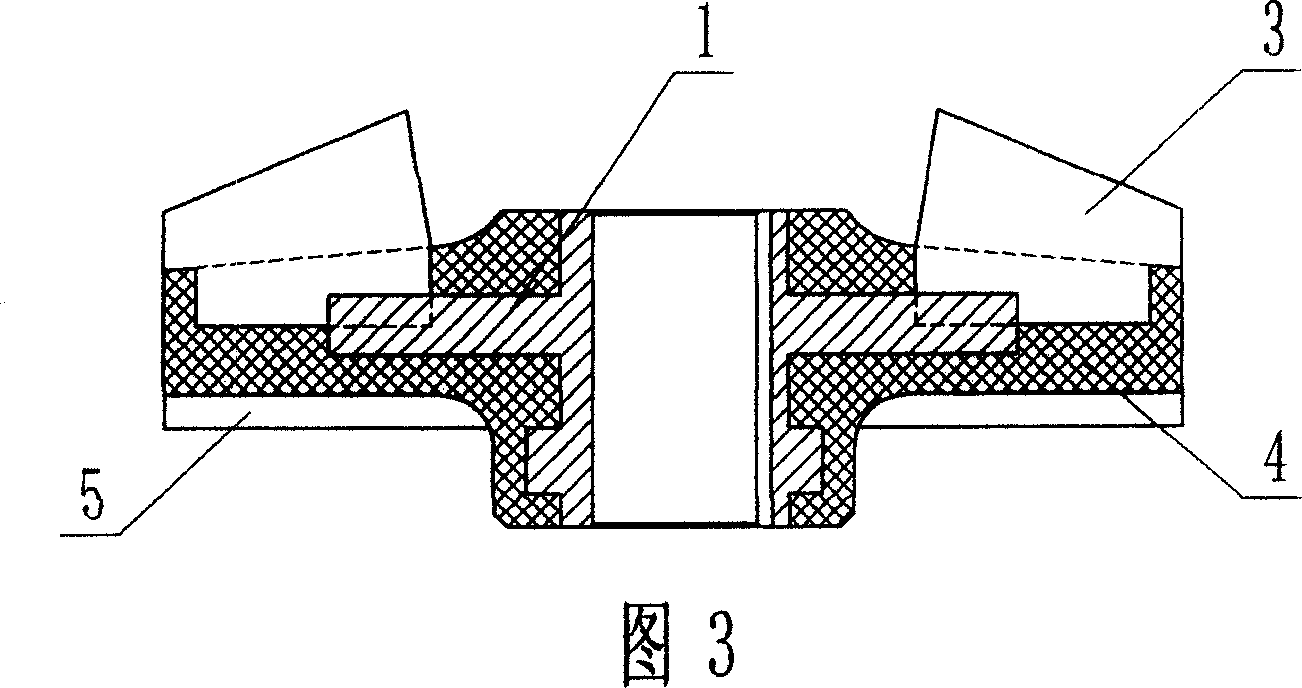
The invention discloses a zero-discharging technology in reclaimed water reuse. A biochemical water-discharging tank which is used for accommodating biochemically discharged water is arranged. An ultrafiltration device, a roll-type reverse osmosis membrane device, a super reverse osmosis membrane device and an evaporation device are successively arranged on the biochemical water-discharging tank.A clean water tank is connected to the roll-type reverse osmosis membrane device, the super reverse osmosis membrane device and the evaporation device. The invention discloses a super reverse osmosismembrane device which includes a circular tube pressure bearing outer shell, a metal sealing piece, a flange disk, a fluid reversing disk, a sealed circular tube pressure bearing inner shell, a waterdistributer, an inner pulling rod, a supporting and guiding disk, a membrane piece, a liquid inlet pipe and a liquid outlet pipe. The invention provides the zero-discharging technology in reclaimed water reuse, which can reduce energy consumption of sewage treatment and further reduce investment required in the sewage treatment. By means of the technology, a sewage treatment effect is improved anda zero-discharging requirement is achieved.
Owner:CHENGDU MEIFUTE MEMBRANE TECHNOLOGY CO LTD
Separating and filtering membrane column
InactiveCN104174292ASmall pressure lossReduce stressSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisSewageEngineering
The invention discloses a separating and filtering membrane column. The separating and filtering membrane column comprises a hollow cylindrical pressure-bearing shell, at least two guide discs and at least one diaphragm are arranged in the pressure-bearing shell, a central pull rod is also arranged at the middle positions of the guide disc and the diaphragm for fixation, a stacking plate, a fluid reserving disc and a flange plate are arranged at each of the upper end and the lower end of the pressure-bearing shell from inside to outside symmetrically, the flange plates seal the upper end and the lower end of the pressure-bearing shell, and an outer pull rod for tensioning the flange plate on the pressure-bearing shell is also arranged on the flange plate. According to the separating and filtering membrane column, the pressure loss is further reduced, the sewage purifying efficiency is improved, the using effect is also improved, good market recognition is achieved, industrial development is promoted and the competitive capacity of enterprises is improved.
Owner:CHENGDU MEIFUTE MEMBRANE TECHNOLOGY CO LTD
Mooring anchor leg replacement method
The invention discloses a mooring anchor leg replacement method comprising the following steps: adjusting the heading of a floating production storage and offloading oil tank; installing an anchor point of a new anchor leg through an anchor point installation vessel, and laying an anchor head chain and a new lower anchor cable; recycling a new lower anchor cable connector through an anchor chain laying vessel, installing a dynamometer, and tensioning the new anchor leg; recycling an adjustment chain, removing the dynamometer from the adjustment chain, and adjusting the length of the adjustment chain; connecting a new balance weight chain to the adjustment chain, and laying the new balance weight chain on a seabed; cutting an old anchor leg through a saturation diving support vessel and an air diving support vessel, and recycling an old upper anchor cable; and installing a new upper anchor cable, and connecting the installed new upper anchor cable to the new balance weight chain. A mooring old anchor leg can be replaced under in-service production of the floating production storage and offloading oil tank, disengaging and back-jointing of the floating production storage and offloading oil tank are avoided, the workload is greatly reduced, the construction efficiency and reliability are improved, and the yield loss and output loss of oil fields are reduced.
Owner:SHENZHEN OFFSHORE OIL ENG UNDERWATER TECH CO LTD
Auxiliary tool changing device of drum type chipper
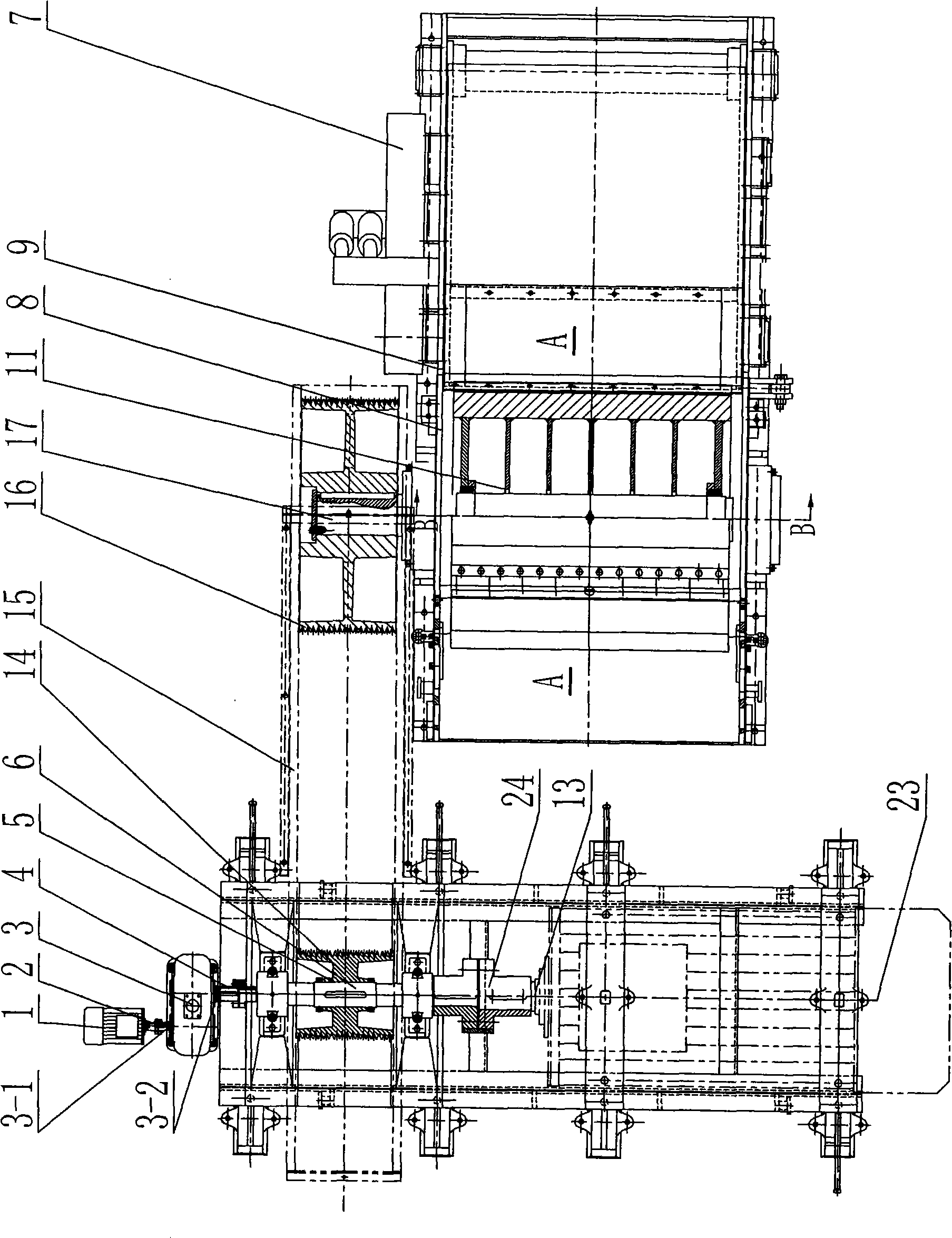
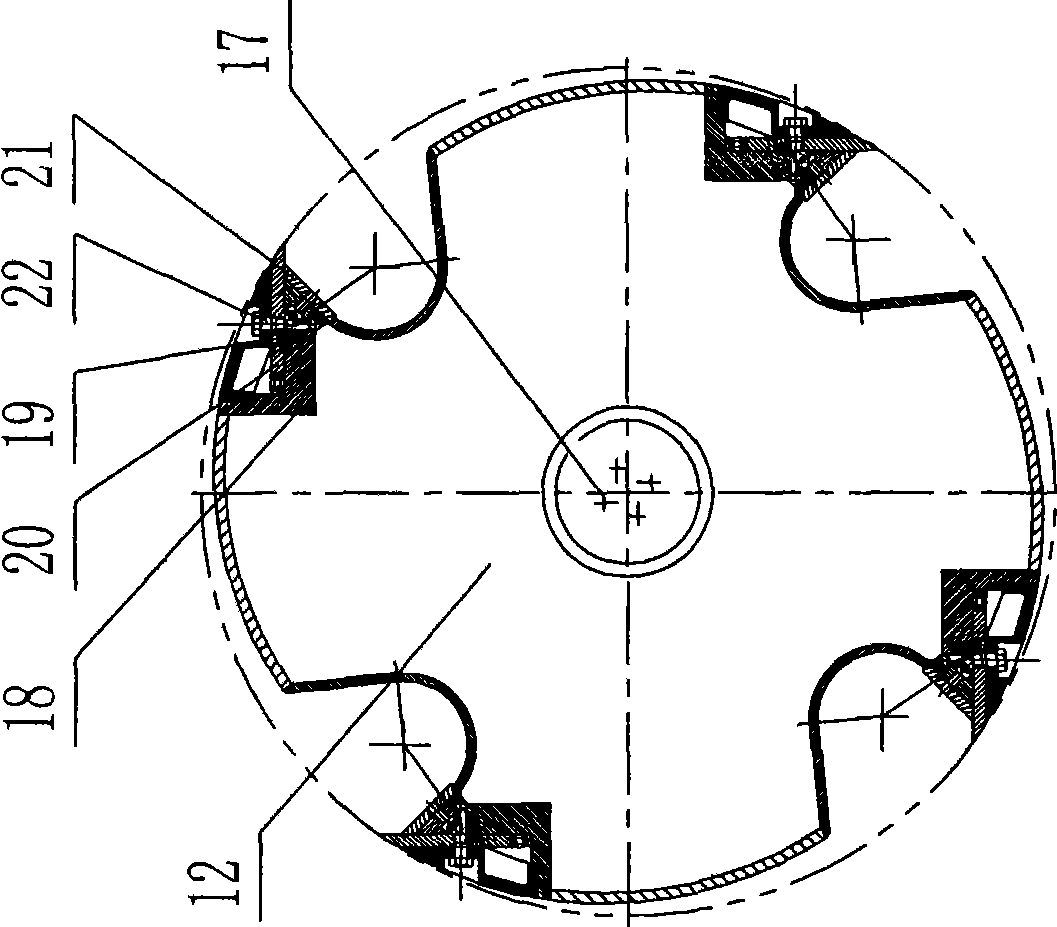
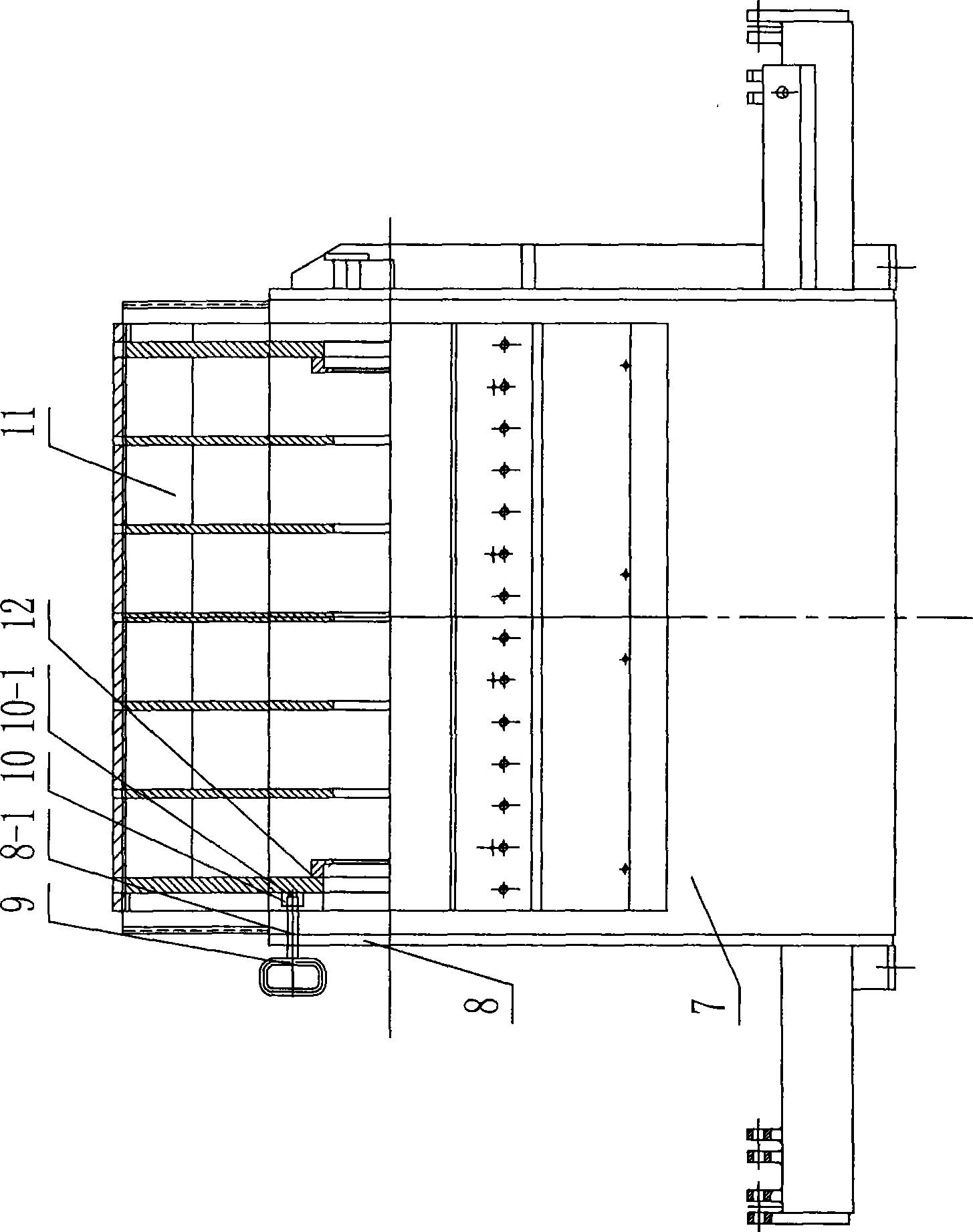
ActiveCN101474815ARealize rotation automationReduce labor intensityVeneer manufactureElectromagnetic clutchCoupling
The present invention relates to a drum chipper, and particularly relates to an auxiliary cutter changing device of drum chipper. The auxiliary cutter changing device of drum chipper comprises an inserted pin (9), a positioning block (10) and a chipper base hole (8-1). The positioning block (10) is installed on a lining board (12) at the end plane of cutter roller (11). A positioning hole (10-1) is provided on the positioning block (10). The chipper base hole (8-1) is installed on a chipper base side plate (8). The auxiliary cutter changing device of drum chipper also comprises an auxiliary cutter changing motor (1), a coupling (2), a speed reducer (3) and an electromagnetic clutch (4). The auxiliary cutter changing motor (1) is connected with an input shaft (3-1) of speed reducer (3) through the coupling (2). The electromagnetic clutch (4) is installed between the output shaft (3-2) of speed reducer (3) and a main shaft (6) of drum chipper driving device (5). The auxiliary cutter changing device of drum chipper according to the invention has the advantages of: (1) time saving, labor saving, and short changing time; (2) greatly increased production efficiency.
Owner:江苏保龙机电制造有限公司
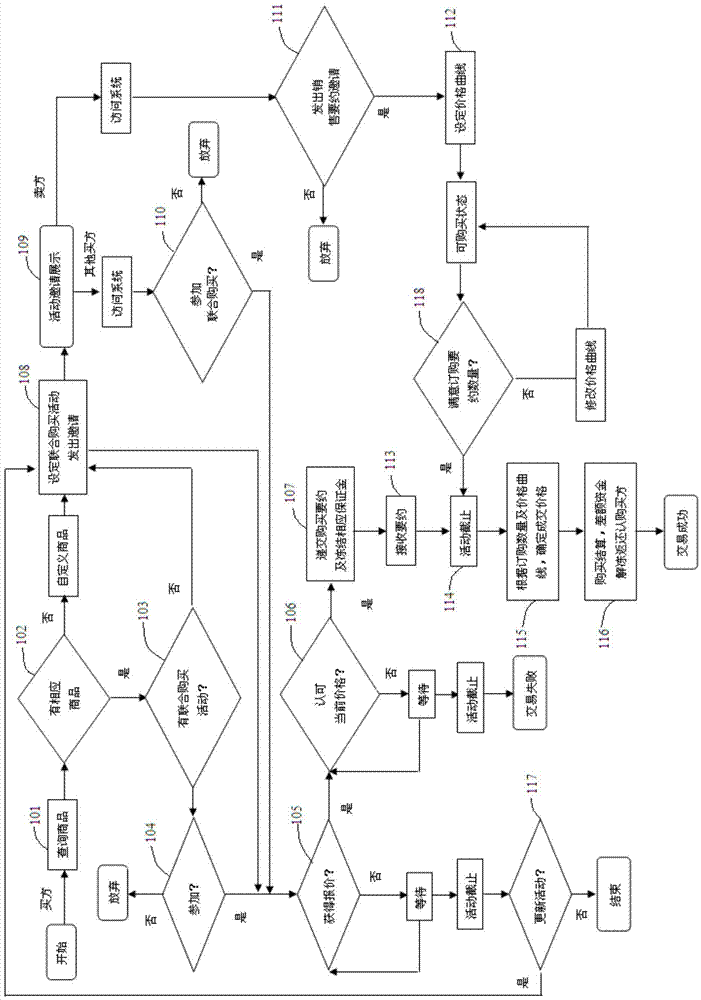
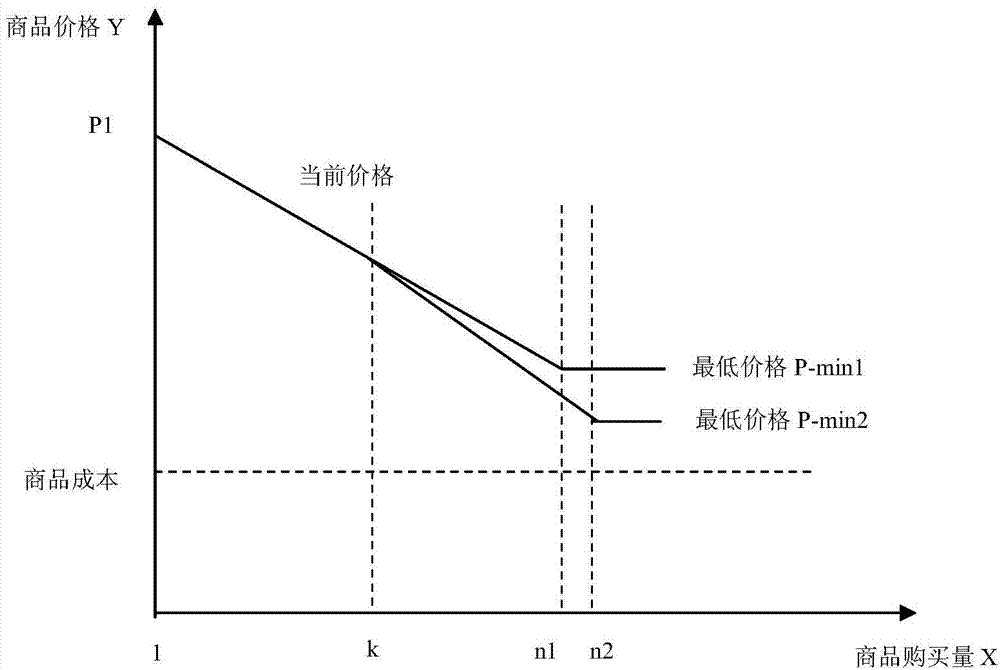
System and method for processing data formed when multiple users co-buy commodities
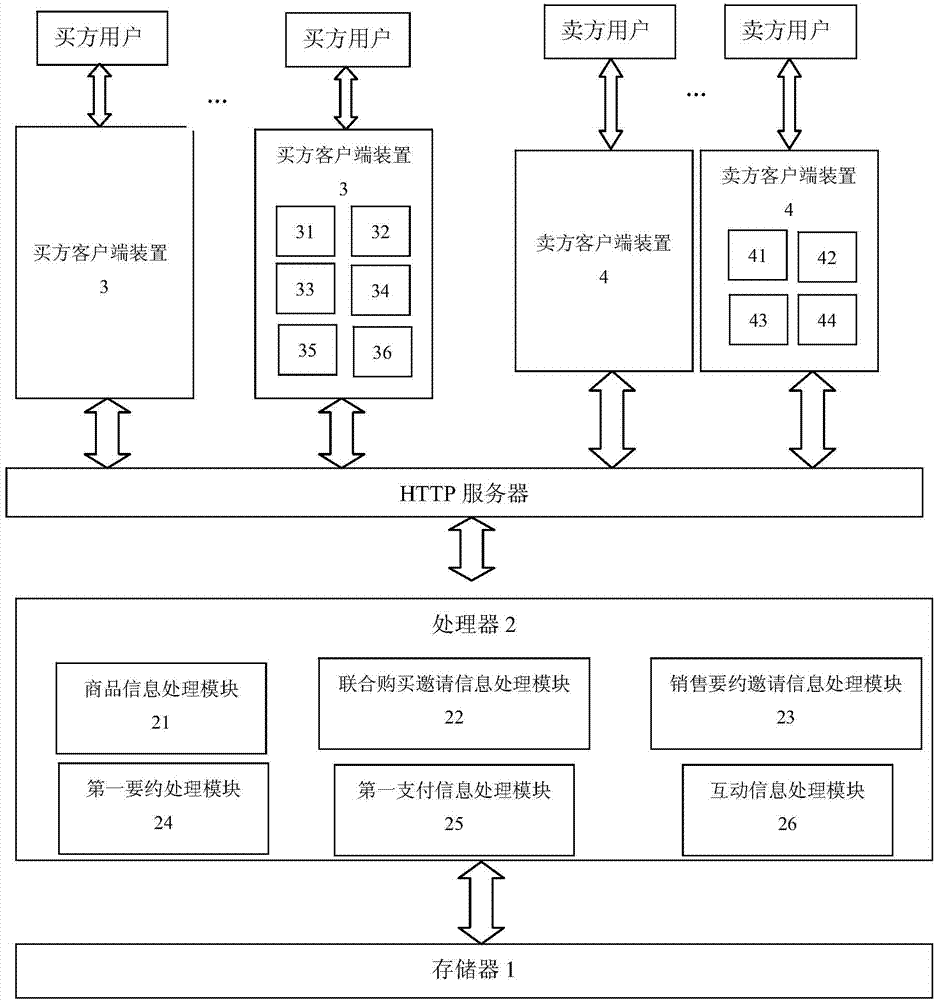
InactiveCN104240120AHelp arrangeSpeed up inventory turnoverBuying/selling/leasing transactionsData processing systemClient-side
The invention relates to a system and a method for processing data formed when multiple users co-buy commodities. By the system and the method, releasing of a co-buy invitation by a buyer and co-buying of a same commodity and completing of a transaction by multiple users are realized. The system comprises a server device, buyer client devices and a seller client device, the server device comprises a storage and a processor in communication with the storage, and both the buyer client devices and the seller client device are in communication connection with the processor. The method includes that one buyer client device sets information of a co-buy activity for a needed commodity and sends the co-buy invitation out through the processor; the processor receives a selling offer invitation sent out by the seller client device; a plurality of the buyer client devices send buying offers to the processor, and the processor sends the buying offers to the seller client device; when the co-buy activity is finished, a final transaction price is generated. Compared with the prior art, the system and the method have the advantages of high interactivity, high feedback speed and the like.
Owner:孟宪强
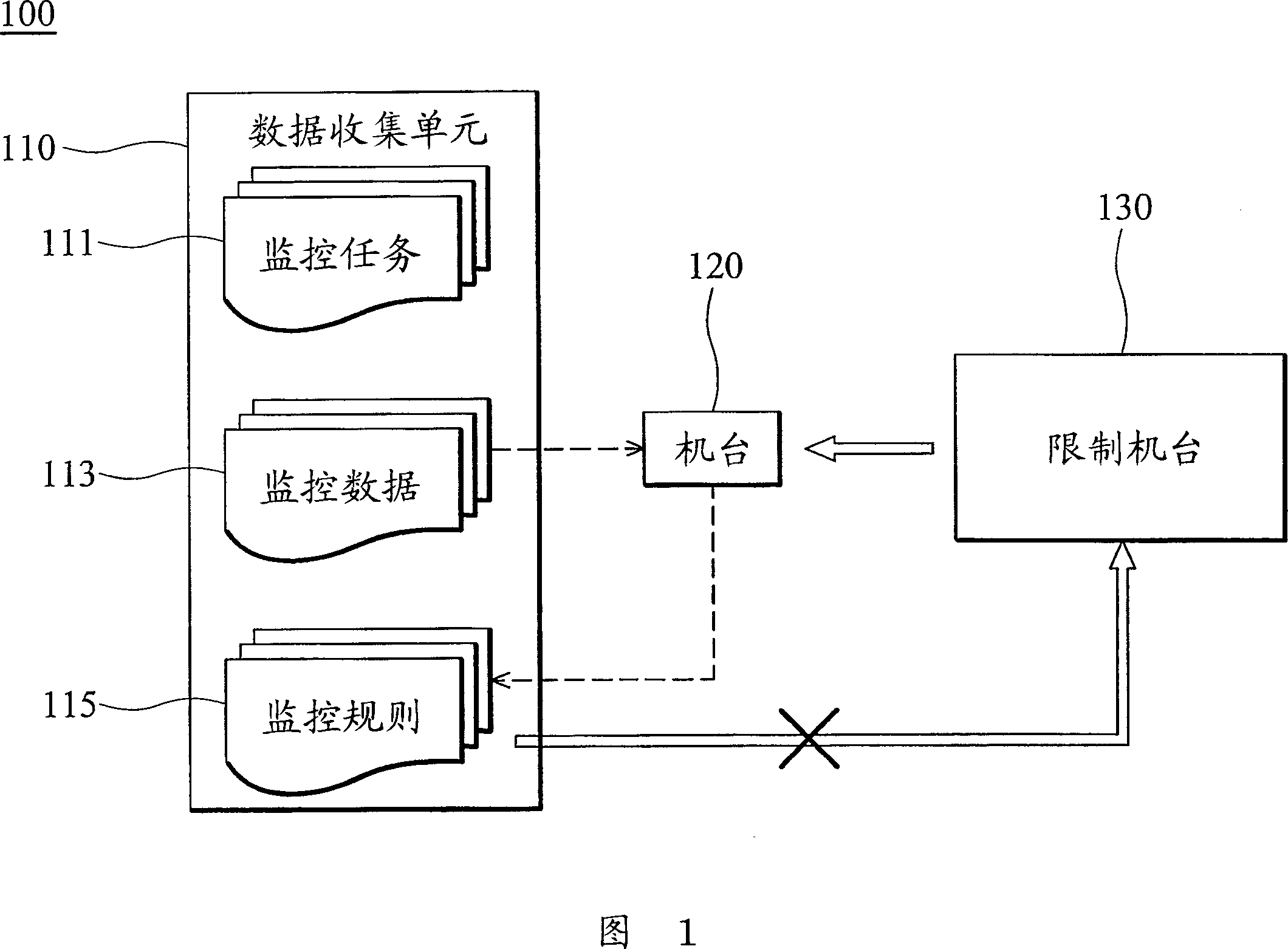
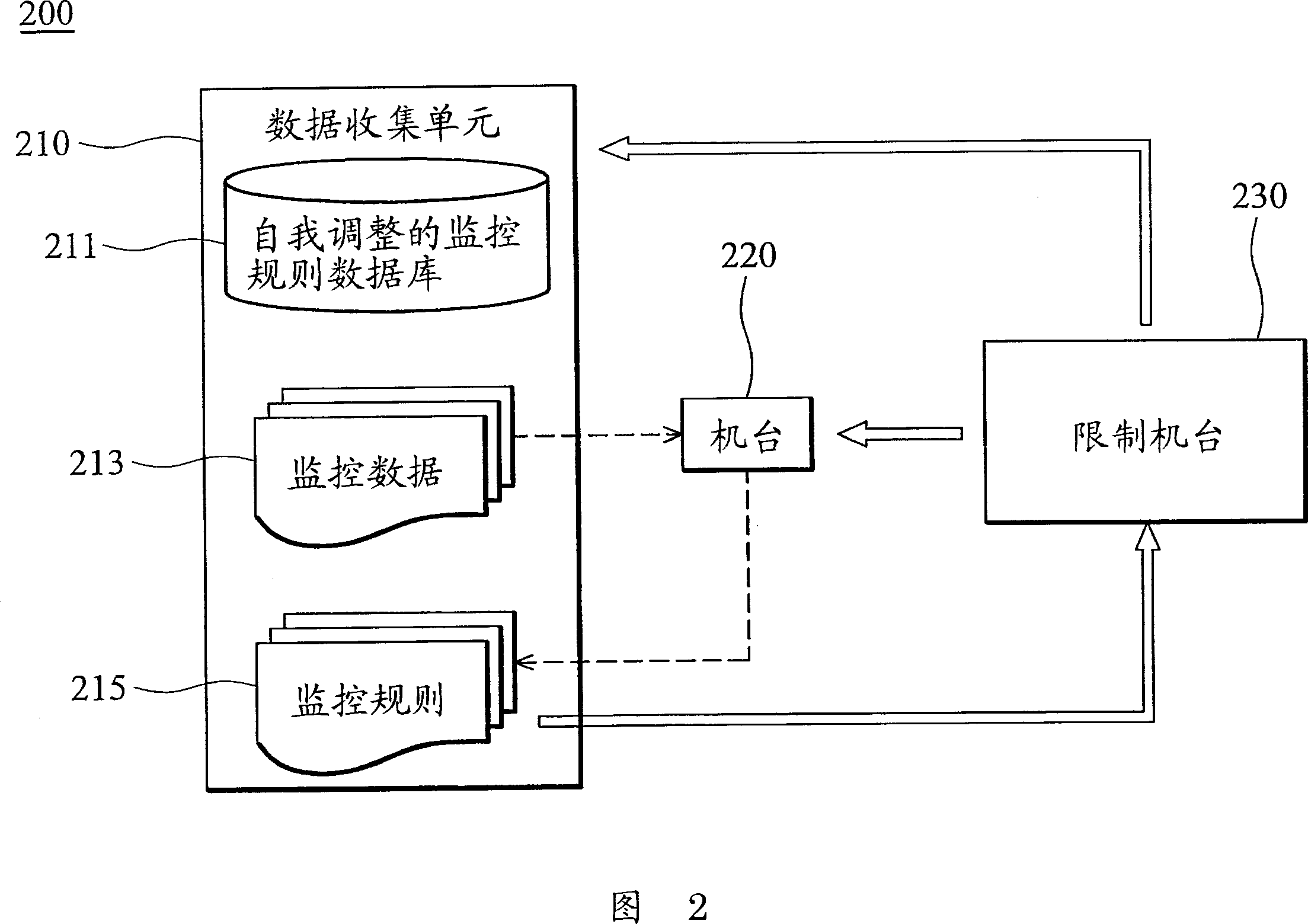
Methods and systems of offline measurement for process tool monitoring
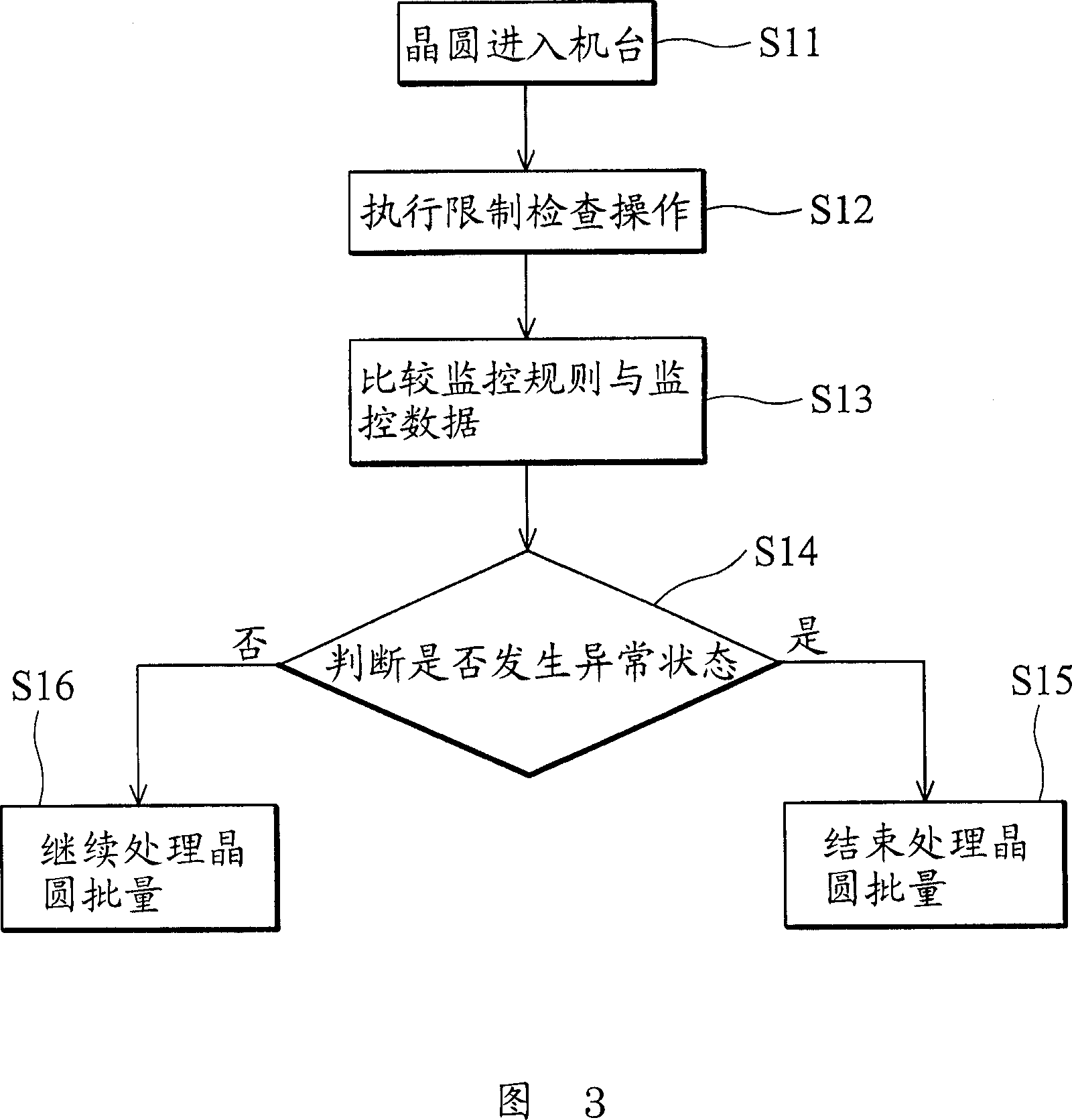
ActiveCN1983089AImprove efficiencySimple processSemiconductor/solid-state device testing/measurementComputer controlSelf-tuningComputer science
The invention provides a method and a system of offline measurement for process tool monitoring. A self-tuning monitor rule database, storing predefined monitor rules for lot processing is provided. Monitor data related to the lot processing is defined. Desired monitor data is obtained according to selected monitor rules residing in the self-tuning monitor rule database. Offline measurement operations are implemented according to the obtained monitor data using a process tool to generate monitor results. It is determined whether abnormal states exist by comparing the selected monitor rules and monitor data according to the monitor results. If so, the lot processing for the process tool is terminated. If not, the lot processing for the process tool is allowed. A failure notice is sent in response and the selected monitor rules are re-defined to update the self-tuning monitor rule database. The invention can improve the efficiency and flow of the lot processing, reduce the loss of production.
Owner:TAIWAN SEMICON MFG CO LTD
Anti-electrostatic heater plate structure
InactiveCN104253066AEliminate static electricityImprove fragmentation problemSemiconductor/solid-state device manufacturingPhotomechanical coating apparatusRadiationDisplay device
The invention provides an anti-electrostatic heater plate structure applied to photoresist coating of flat-plate displays and developer tables. A glass substrate is arranged on the top surface of a heater plate and is heated up by the same. A plurality of jacking rods capable of jacking upwards from the top surface of the heater plate are mounted in the heater plate. A plurality of fixed jacking pins arranged at intervals are disposed on the top surface of the heater plate and used for supporting the glass substrate on the top surface of the heater plate. Compared with the conventional manner of directly attaching the glass substrate onto the surface of the heater plate, the manner of utilizing the jacking pins to jack the glass substrate by 0.1MM can not only eliminate electrostatic influences of the heater plate but eliminate influences to heat radiation conduction.
Owner:EVERDISPLAY OPTRONICS (SHANGHAI) CO LTD
Welding process of plug-in unit of PCB (Printed Circuit Board)
InactiveCN102950349AImprove protectionImprove securitySoldering auxillary devicesVisual inspectionEngineering
The invention relates to the technical field of welding of a PCB (Printed Circuit Board), and provides a welding process of a plug-in unit of the PCB, which can effectively improve the production efficiency, improves the production quality, reduces the production loss and cost, and reduces the operation difficulty and human errors. The process comprises the following steps of: (1) trimming supporting legs of components: trimming the supporting legs of the components into union and formal specification; (2) inserting the components: respectively the components processed in step (1) into corresponding welding pads of the PCB on a tray of a wave soldering machine; (3) viewing and pressing the components: performing visual inspection to confirm that the components are correctly inserted, and then pressing the components on the PCB; (4) performing wave soldering welding: performing welding operation on the PCB by the wave soldering machine; (5) taking out the PCB: taking out the PCB from the tray of the wave soldering machine; (6) detection: detecting the welding quality of the PCB, if the welding quality is qualified, obtaining the finished product, and if the welding quality is disqualified, performing the next step on the disqualified product; and (7) repairing welding: performing manual repairing welding operation on detected poor welding points of the PCB, and obtaining the finished product.
Owner:QIXIANG ELECTRON SCI & TECH
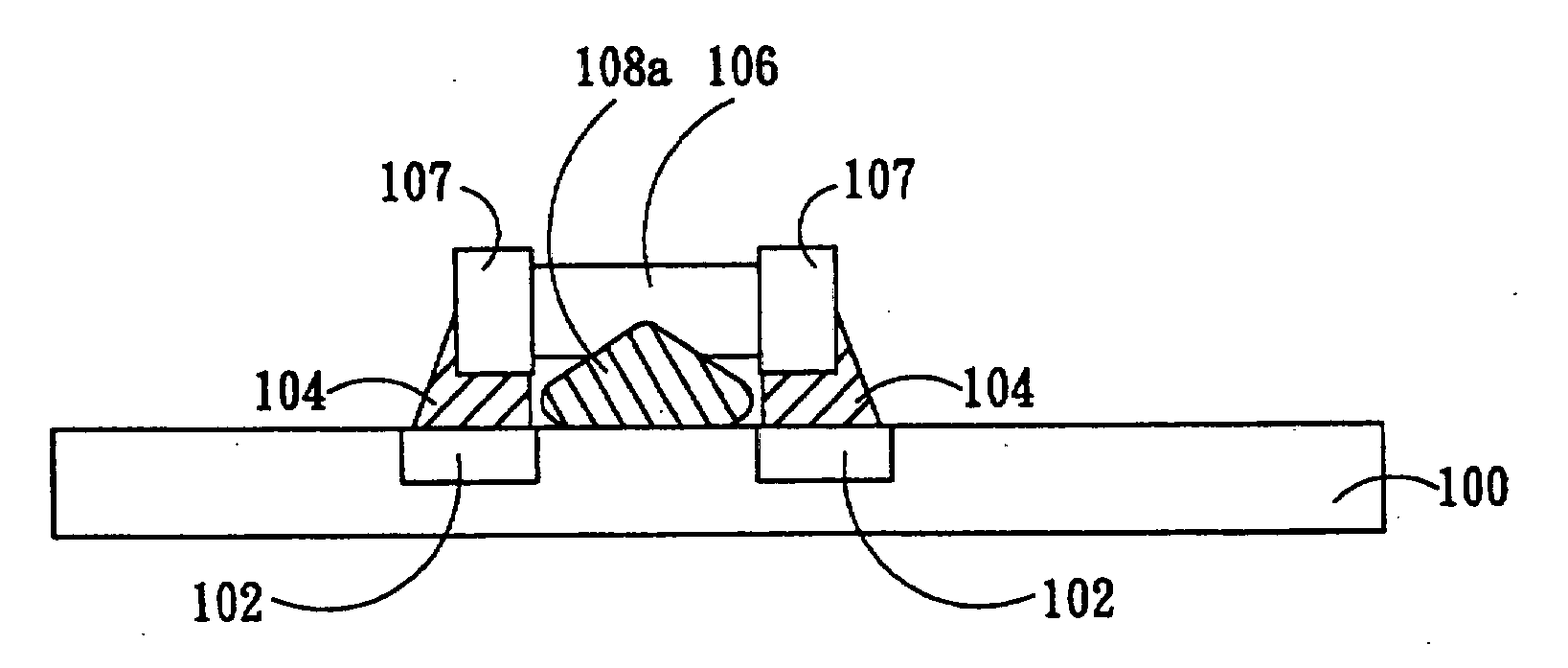
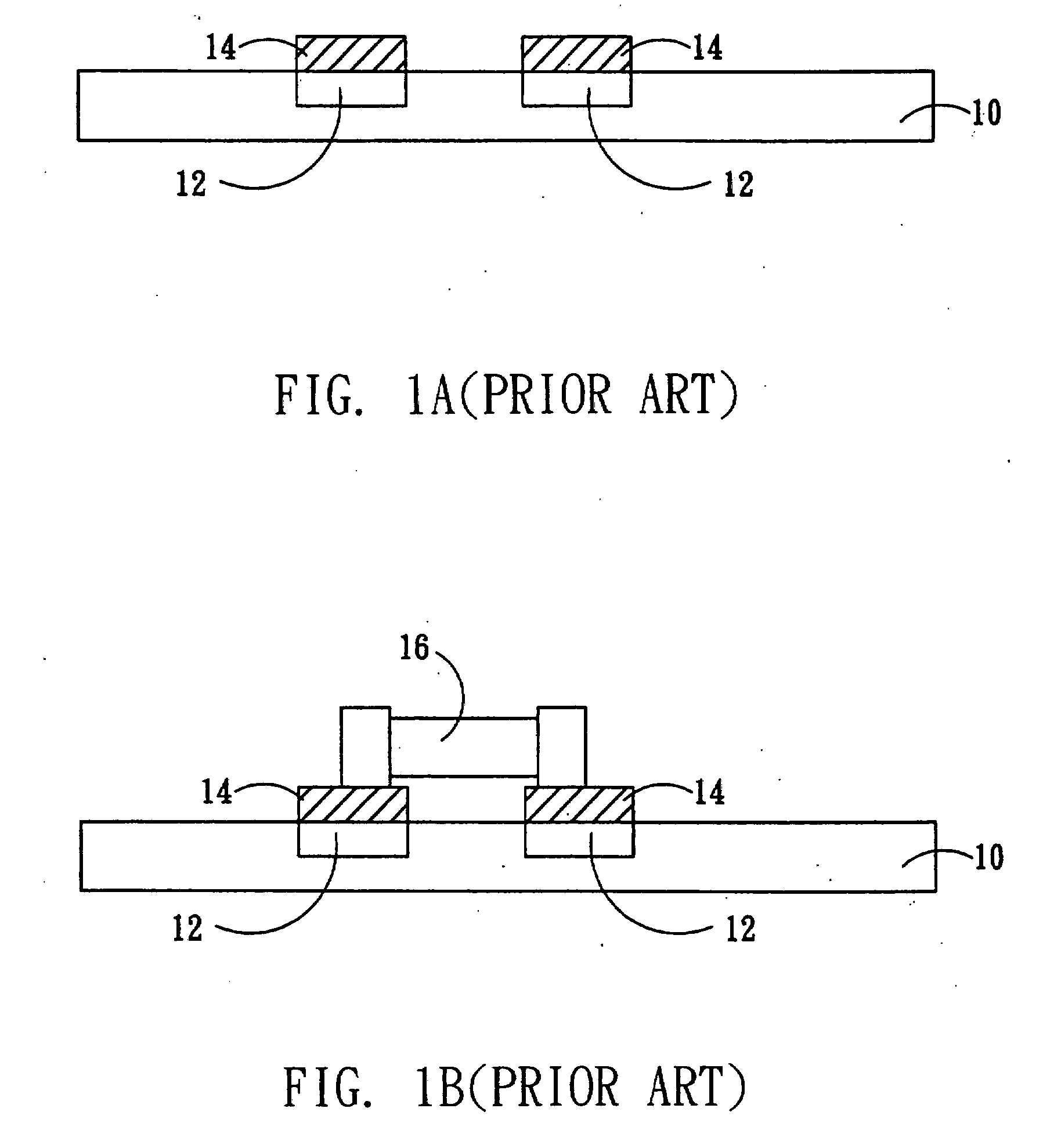
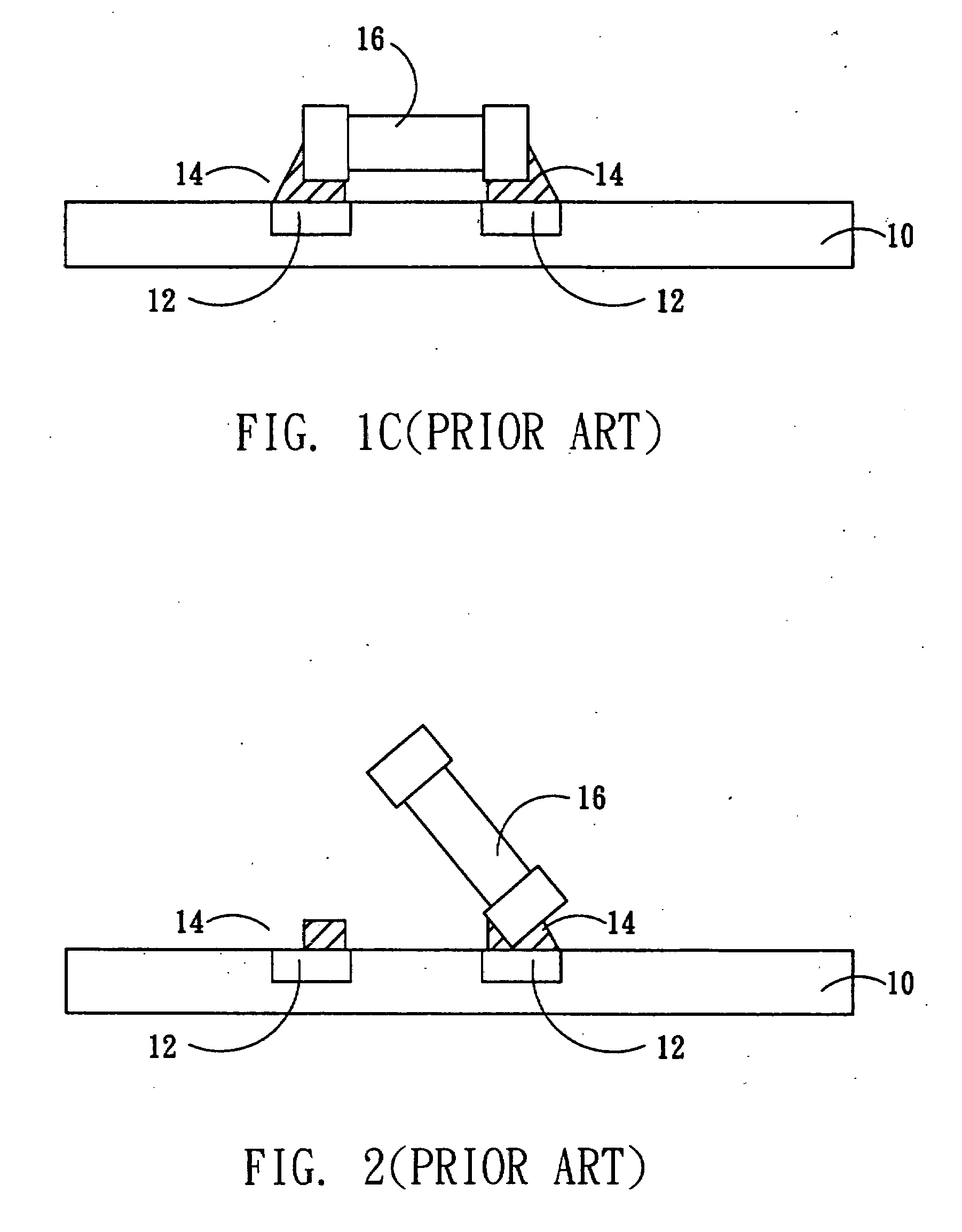
Mounting method of passive component
InactiveUS20070234561A1Easing yield rateReduce production lossPrinted circuit assemblingLine/current collector detailsPull forceEngineering
The present invention relates to a mounting method of passive component, and particularly relates to a mounting method of a small-size passive component. A passive component partially covered by an adhesive material is provided. Before the adhesive layer is melted, the adhesive material is melted and then solidified to form a fixing structure between the passive component and a substrate for fixing the passive component on the substrate. Therefore, the tombstone problem caused by uneven pulling force that the melted solder applies to the two ends of the passive component is effectively solved. The yield rate is increased, and the product loss is reduced.
Owner:ADVANCED SEMICON ENG INC
Manufacturing method of Roselle fig vinegar
The invention discloses a manufacturing method of a Roselle fig vinegar. In the method, apple and roselle are taken as raw materials, ground, stirred, fermented; then vinegar is added and the mixture is finally sterilized. The product of the invention features refreshing taste, strong fruit aroma and has the functions of promoting metabolism, cooling and refreshing, boosting spirit, helping produce saliva and slake thirst, improving digestion, facilitating diuresis and removing edema, nourishing and invigorating blood, beautifying faces and eliminating hangover. Constant taking of the product is conducive to reducing cholesterol and triglyceride, thus achieving the effect of preventing cardiovascular diseases and helping reduce weight; in the invention, fruit use ratio is improved and economic benefits are increased.
Owner:唐文锋
Silicon wafer carrier welding strength testing machine
The invention discloses a silicon wafer carrier welding strength testing machine comprising a machine frame, a gas cylinder mounted on the machine frame, guide rails, and ejector blocks in transmission connection with the gas cylinder; the machine frame is provided with a test clamping position for placing a silicon wafer carrier; the gas cylinder, the guide rails and the ejector blocks are located at one side of the test clamping position; the guide rails extend along the transverse direction; the ejector blocks can move transversely along the guide rails under driving of the gas cylinder to push and press the silicon wafer carrier in the test clamping position so as to realize a silicon wafer carrier welding strength test. The pushing force generated by the gas cylinder is adopted for testing the silicon wafer carrier welding strength, the quality of the silicon wafer carrier is tested, the silicon wafer carrier is ensured to be not damaged when in use, not only is the loss of production reduced, but also the personal safety is ensured; and the silicon wafer carrier welding strength testing machine has the advantages of simple structure and convenient operation, and can be widely popularized and used in the industry.
Owner:JA SOLAR
Method for preparing dapoxetine hydrochloride
ActiveCN105061230ASolve the problem of serious lossEasy accessOrganic compound preparationAmino-hyroxy compound preparationBenproperinePhenylalanine
The invention relates to the field of medicines, and in particular relates to a method for preparing dapoxetine hydrochloride. The method comprises the following concrete steps: (1) by taking chiral L-phenylalanine hydrochloride as a starting material, and reducing by adopting a reducing system to obtain an intermediate II; (2) performing Eschweiler-Clark reaction on the intermediate II and paraformaldehyde or formaldehyde to obtain an intermediate III; and (3) performing Williamson aethrization reaction on the intermediate III and 1-naphthaline to obtain the dapoxetine hydrochloride. According to the invention, chiral L-phenylalanine hydrochloride is used as the raw material, so resolution of a product is avoided, the raw material is easily obtained and relatively low in price, conventional equipment can meet production requirements, expensive and virulent reagents are not used, reaction conditions are mild, pollution is little, control is easy and industrialized production is facilitated.
Owner:KAMP PHARMA
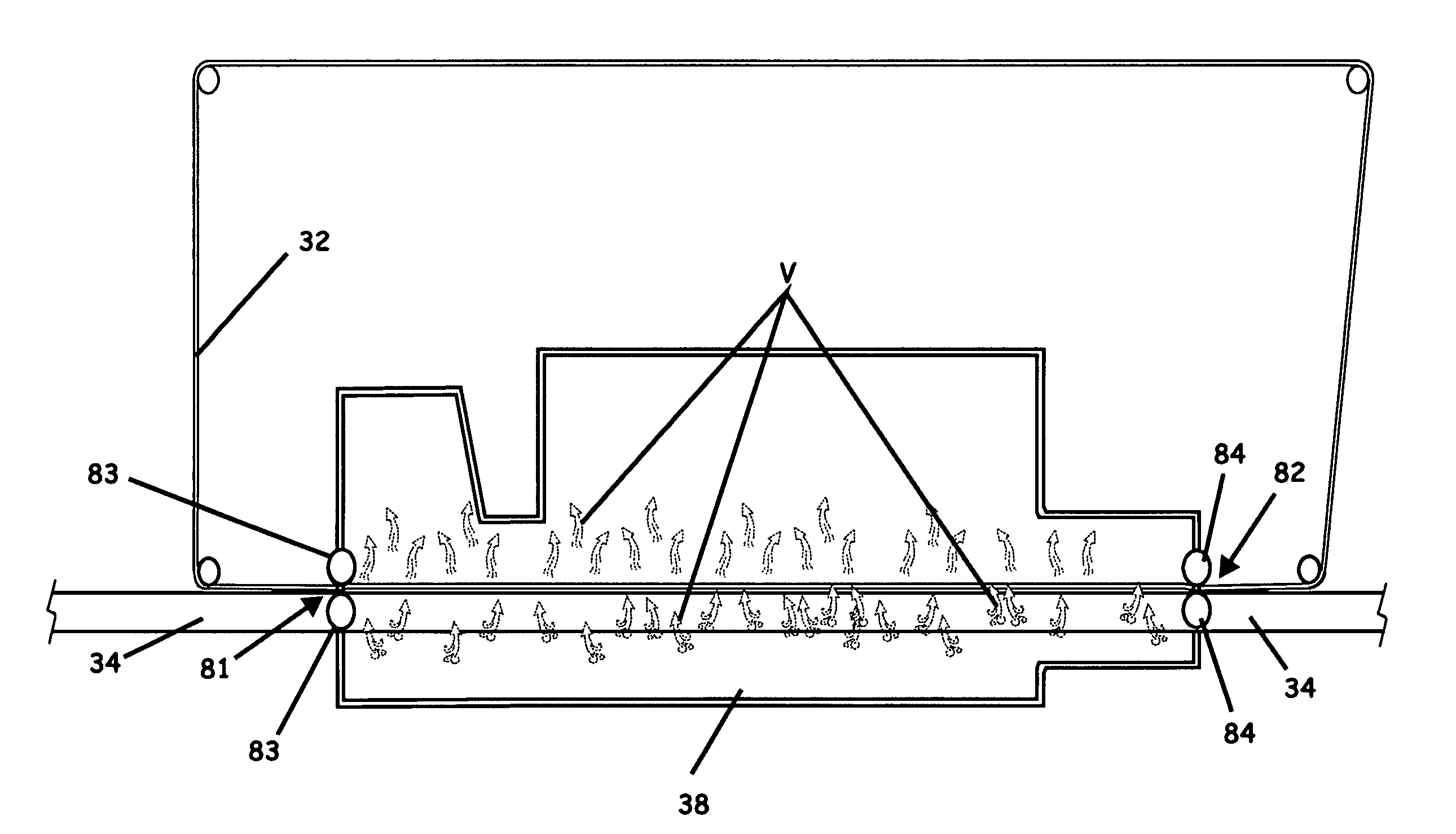
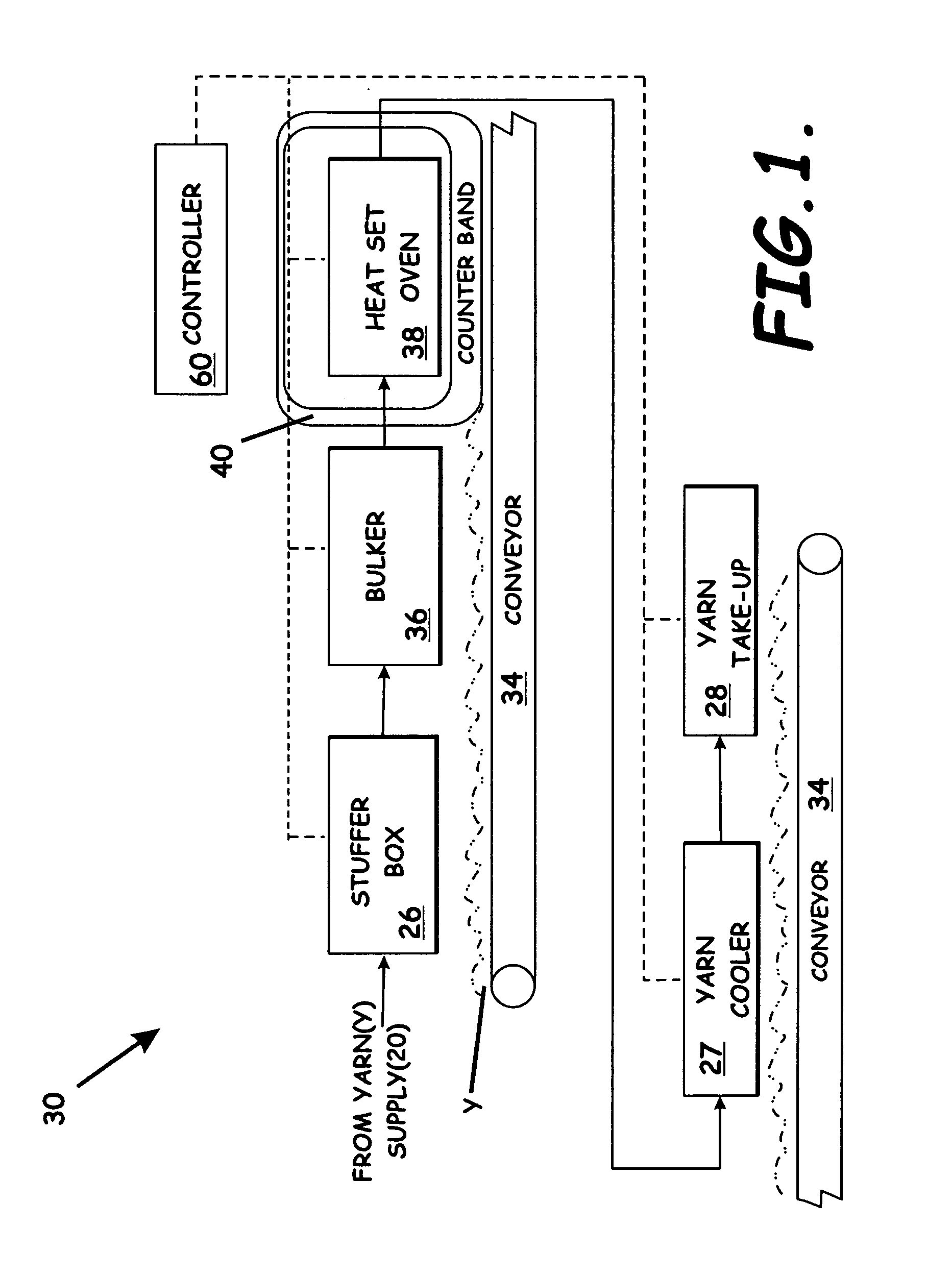
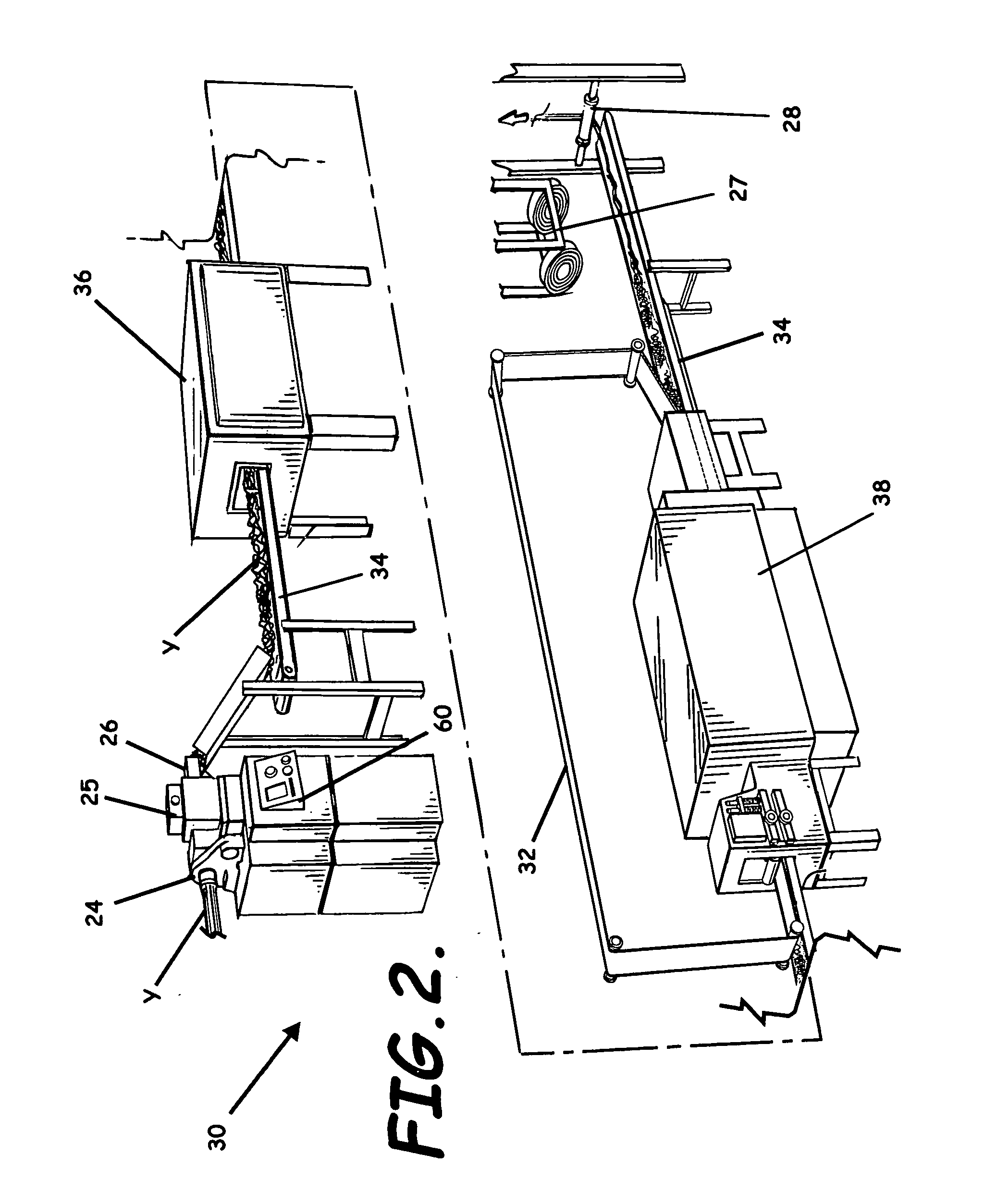
System, apparatus, and method of reducing production loss having a counterband
A yarn system, apparatus, and methods of reducing production loss or increasing production speed in a continuous yarn production process are provided. A yarn conveyor receives a bundle of yarn, an oven heats the yarn, and a yarn cooler cools the yarn exiting the oven to heat set the yarn. The conveyor carries the yarn through the oven and cooler. A counterband extends through the oven substantially parallel to and above the conveyor to abuttingly contact the yarn against the conveyor. The counterband is formed by hydrophobic fibers woven in a mesh pattern that form apertures to enhance flow of a heated fluid between the fibers. A cooling efficiency of the cooler is increased responsive to the hydrophobic fibers allowing the cooler to cool the yarn without moisture from the heated fluid being absorbed by the counterband.
Owner:AMERICAN LINC LLC
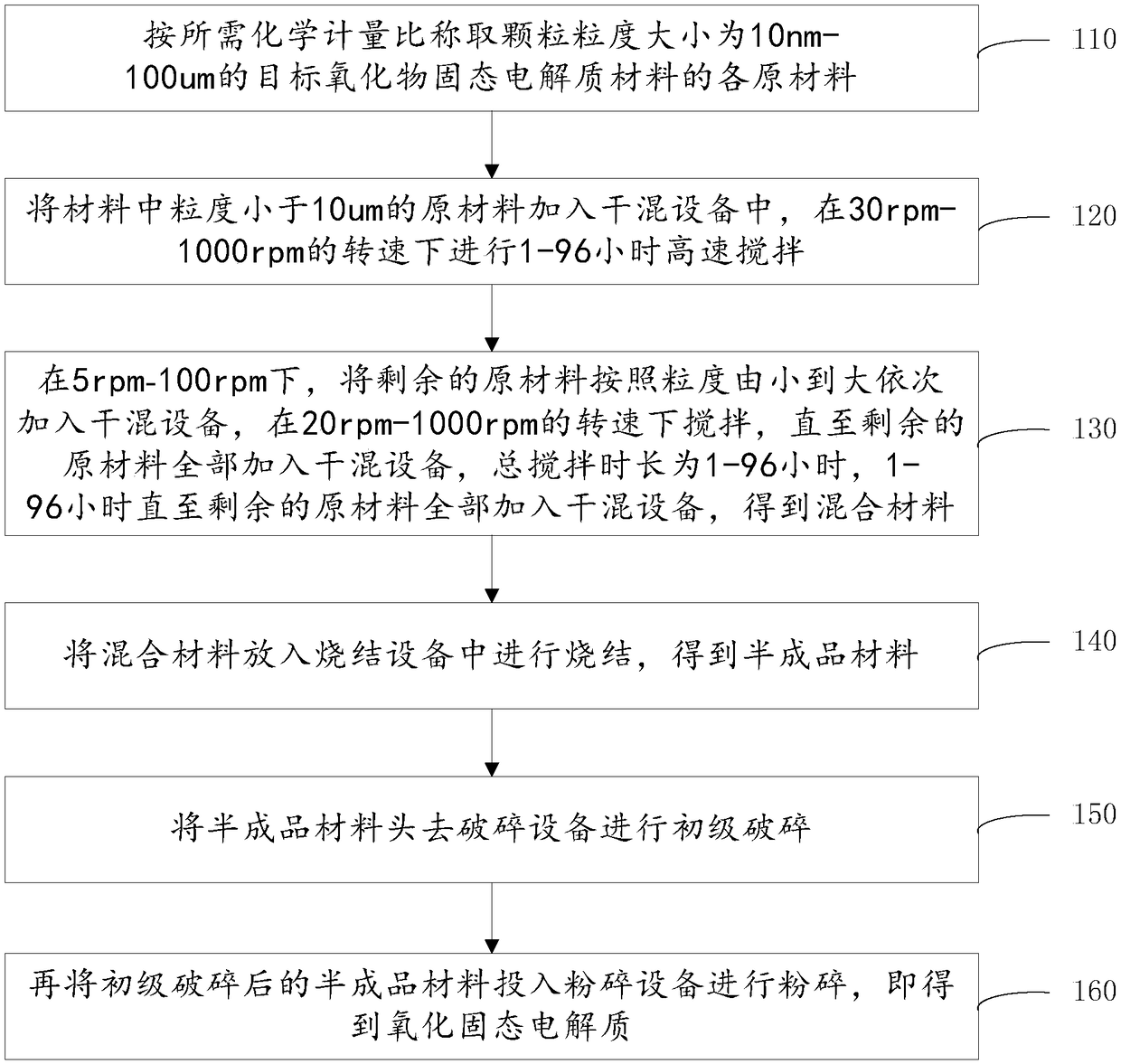
Dry preparation method for oxidized solid electrolyte and oxidized solid electrolyte
The invention discloses a dry preparation method for an oxidized solid electrolyte and an oxide solid electrolyte material. The method comprises the following steps: raw materials, with grain size being 10 nm-100 mu m, of a target oxide solid electrolyte material are weighed in a required stoichiometric ratio; the raw materials with grain size smaller than 10 mu m are added to dry blending equipment and stirred quickly at the rotating speed of 10-1000 rpm for 1-96 h; the remaining raw materials are added to the dry blending equipment from small grain size to large grain size sequentially at the rotating speed of 5-100 rpm, the materials are stirred at the rotating speed of 10-1000 rpm until all the remaining raw materials are added to the dry blending equipment, and a mixed material is obtained; the mixed material is sintered in sintering equipment, and a semi-finished material is obtained; the semi-finished material is fed to crushing equipment for preliminary crushing; the preliminarily crushed semi-finished material is fed to smashing equipment for smashing, and the oxidized solid electrolyte is obtained.
Owner:LIYANG TIANMU PILOT BATTERY MATERIAL TECH CO LTD +1
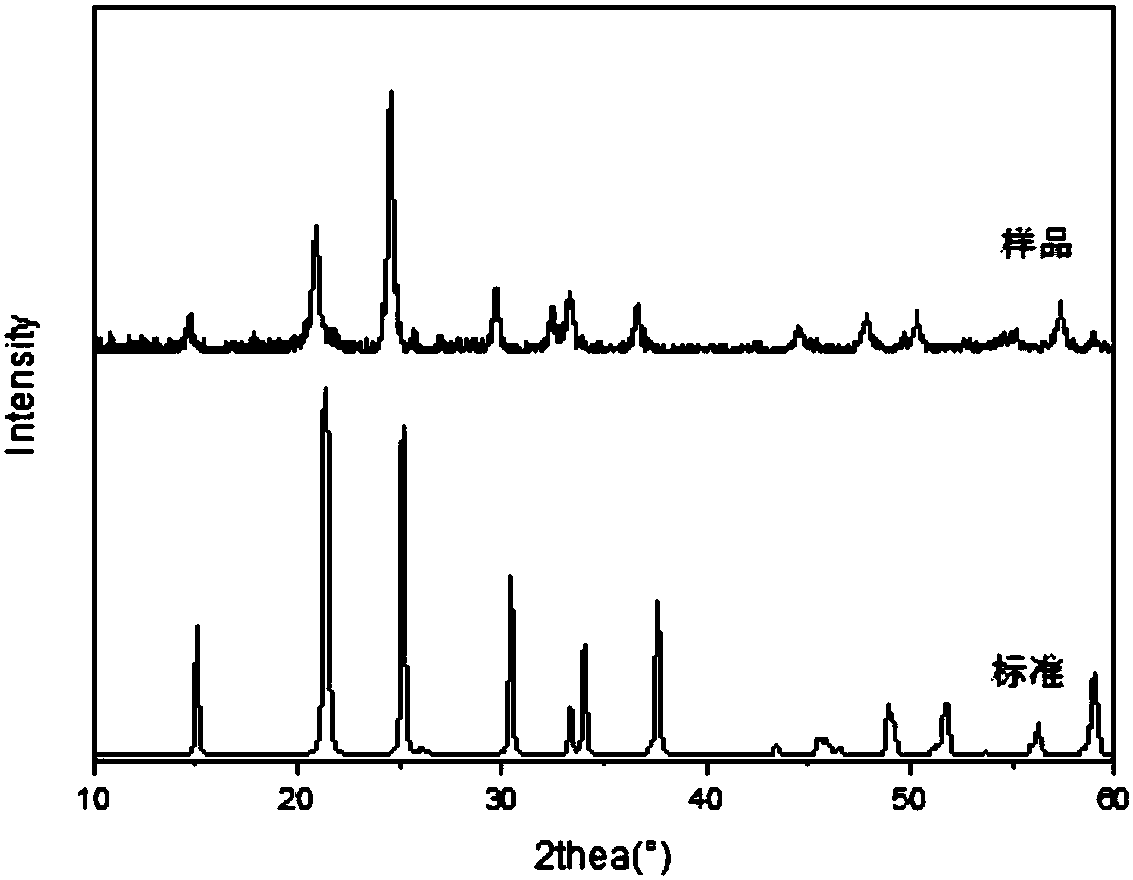
System and method for producing fracturing propping agent by taking red mud as raw material
The invention discloses a system and a method for producing a fracturing propping agent by taking red mud as a raw material. The system comprises a charging machine, a high temperature furnace, a swing-out device, a blanking bin, a tempering furnace and screening equipment, wherein the high temperature furnace is connected with the blanking bin or the swing-out device; dosing is performed by taking inorganic solid wastes as raw materials; and the product is prepared by performing high-temperature melting in the high temperature furnace and molding in a molding device. The method comprises the following steps: (1) dosing, namely mixing pure red mud or red mud with one or more of fluorite, silica sand, potassium (or sodium) feldspar and dolomite; (2) mixing and performing wet grinding, namely mixing and performing wet grinding on the prepared materials; (3) drying and granulating; (4) melting the granulated materials in the high temperature furnace; (5) molding the granulated materials into propping agent granules by utilizing the molding device; and (6) screening, weighing and packaging the propping agent granules by virtue of the screening equipment. The system and the method are high in product yield, high in production efficiency and low in cost.
Owner:JINGANG NEW MATERIALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com