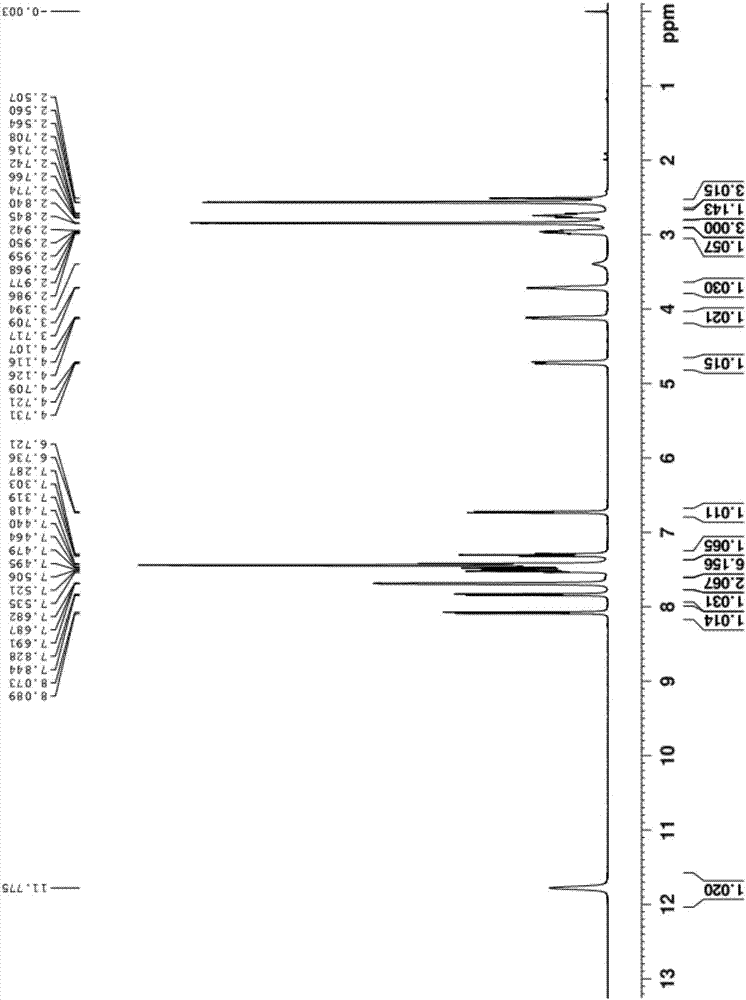
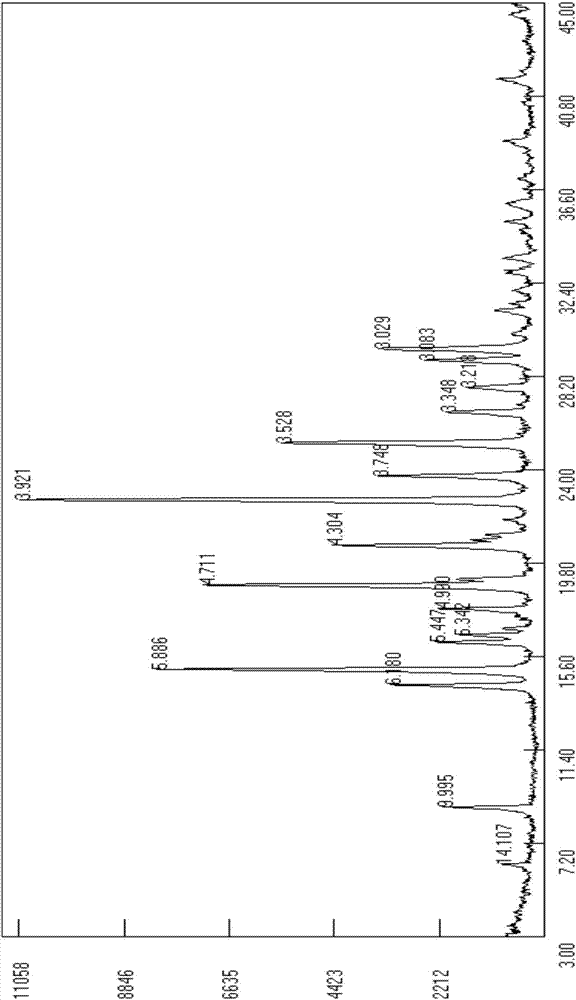
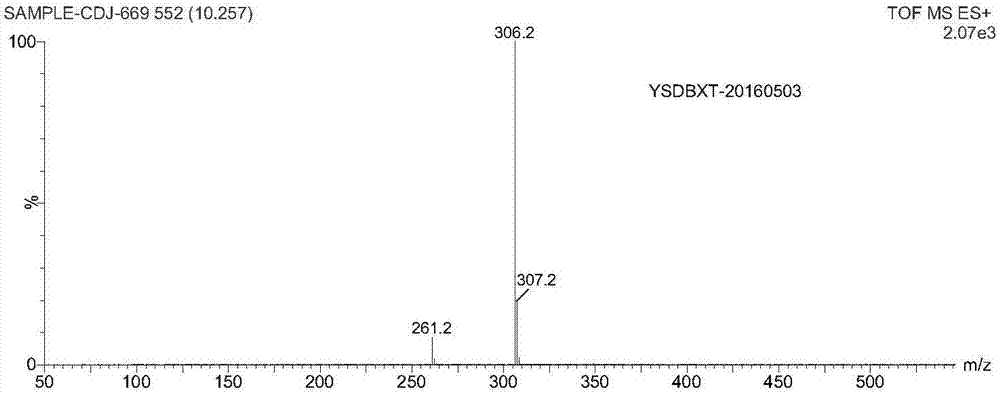
Method for preparing dapoxetine hydrochloride
A technology of dapoxetine hydrochloride and amino, which is applied in the field of preparation of dapoxetine hydrochloride, and can solve problems such as difficult control of hydrochloric acid dosage, high safety and protection requirements, and unfavorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Disperse 1.8kg (10.90mol) (s) 3-amino-3-phenylpropionic acid in 18L tetrahydrofuran, add 1.2kg (32.68mol) sodium borohydride, slowly add 9.1kg (48-50%, 32.68mol ) boron trifluoride-tetrahydrofuran solution, after the dropwise addition, react at 30°C for 5h, stop the reaction, quench the reaction with 9L methanol, concentrate, extract with ethyl acetate 4.8L / time*4 times, combine the ethyl acetate layers, Wash twice with 4.8L saturated saline. Dry and concentrate to give (s) 3-amino-3-phenylpropanol. Dry weight 1.57kg, purity (HPLC): 99.91%, yield 95.35%. Ethyl acetate recovery recycling.
[0098] Weigh 1.5kg (9.9mol) of (S)-3-amino-3-phenylpropanol obtained above and dissolve it in 1.6kg (88%, 30mol) of formic acid, add 2.8kg (37%, 35mol) of formaldehyde The solution was heated to reflux and reacted for 8h. Stop the reaction, concentrate under reduced pressure, add 2L of water, adjust the pH to 12 with 10% aqueous sodium hydroxide solution, and then extract with 3L / ...
Embodiment 2
[0103] Disperse 20.0g (0.11mol) (s) methyl 3-amino-3-phenylpropionate in 200ml tetrahydrofuran, add 12.48g (0.33mol) sodium borohydride, add dropwise 92.34g (50%, 0.33mol) boron trifluoride-tetrahydrofuran solution, control the internal temperature to less than 10°C, after the dropwise addition, react at 30°C for 4h, stop the reaction, add dropwise 100ml of methanol to quench the reaction, concentrate, use 100ml / time*4 times of dichloro After extraction with methane, the organic layer was washed twice with saturated brine and water, dried and concentrated to obtain 15.44 g (s) of 3-amino-3-phenylpropanol. Yield 92.0%, purity (HPLC): 99.82%.
Embodiment 3
[0105] Disperse 10.0g (0.060mol) (s) 3-amino-3-phenylpropionic acid in 300ml methyl tert-butyl ether, add 6.87g (0.18mol) sodium borohydride in batches, cool to 0°C, drop Add 53.70g (48%, 0.18mol) boron trifluoride-diethyl ether solution, control the internal temperature to be less than 10°C, after the dropwise addition, react at 30°C for 6h, stop the reaction, add dropwise 10ml of ethanol to quench the reaction, concentrate, and use 30ml / time*4 dichloromethane extractions, the organic layer was washed twice with saturated brine and water respectively, dried and concentrated to obtain 8.30 g(s) of 3-amino-3-phenylpropanol. Yield 90.6%, purity 99.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com