Process for the preparation of candesartan cilexetil form i
A technology of candesartan cilexetil and sartan alkyl ester, which is applied in the field of preparation of candesartan cilexetil crystal form I, can solve the problem that small particles are sticky, easy to agglomerate, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
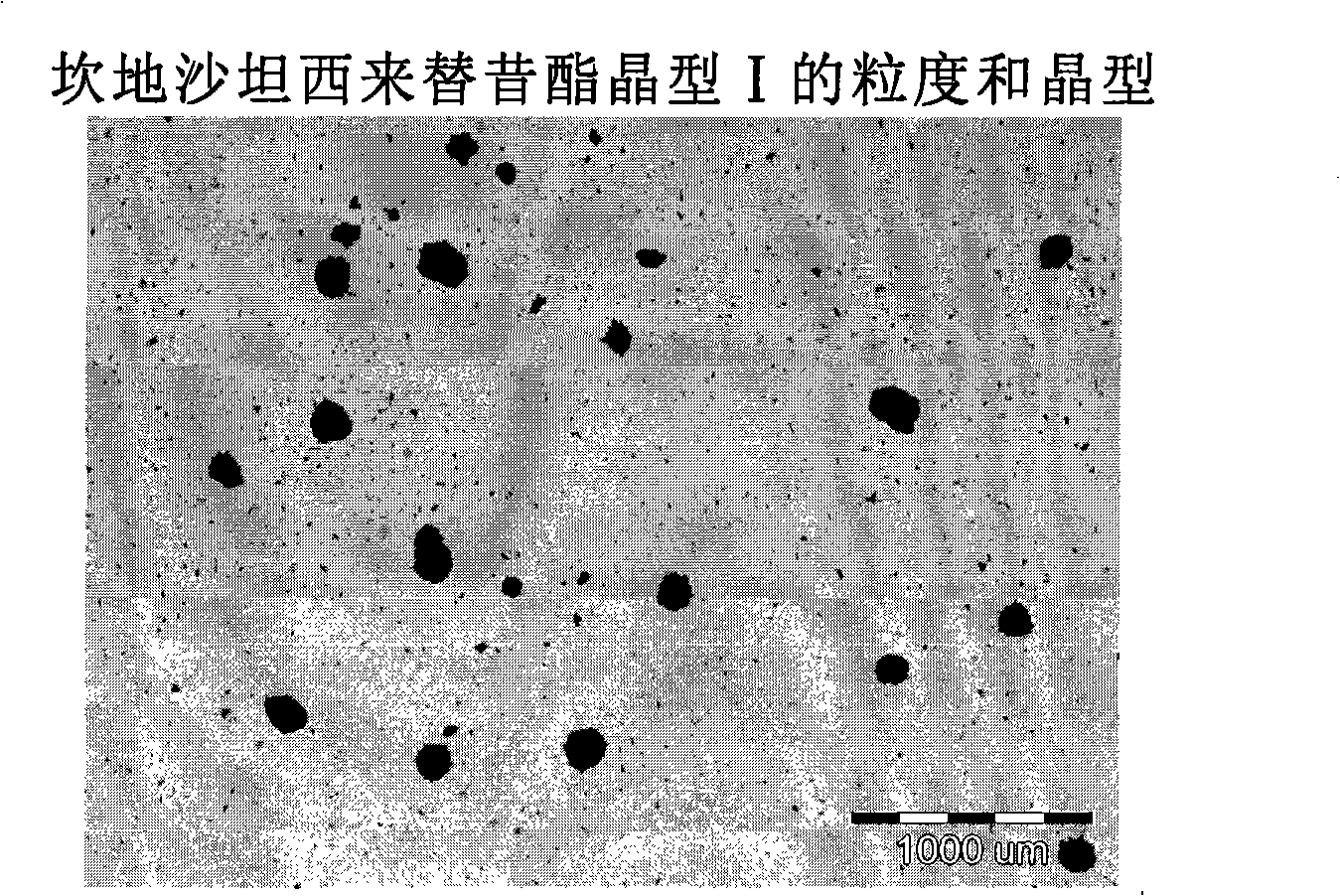
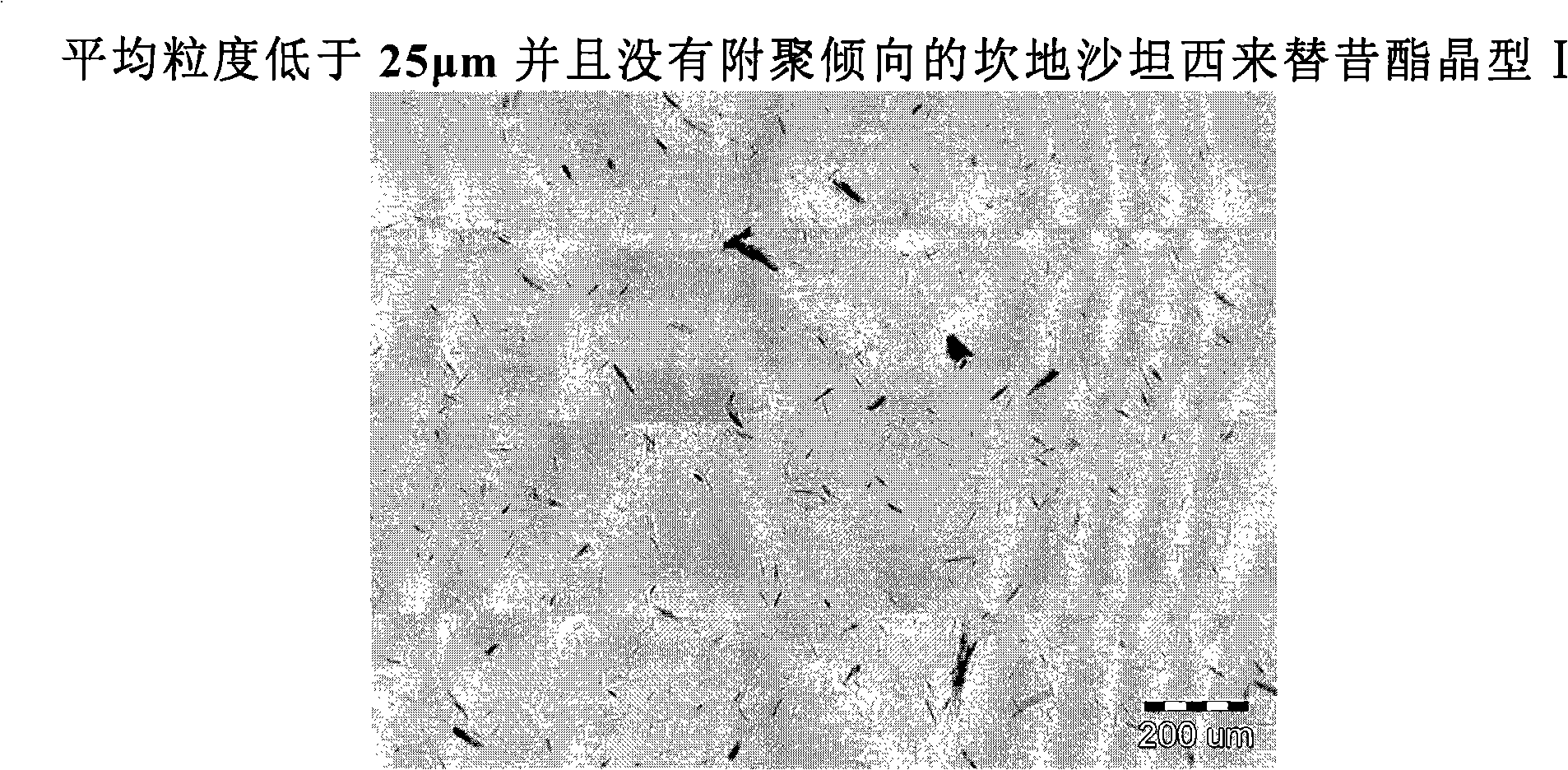
[0022] An object of the present invention is to prepare candesartan cilexetil crystalline form I with an average particle size below 25 μm and no tendency to agglomerate.
[0023] According to a first specific embodiment of the present invention, candesartan cilexetil is prepared by the following method, which method includes the following steps:
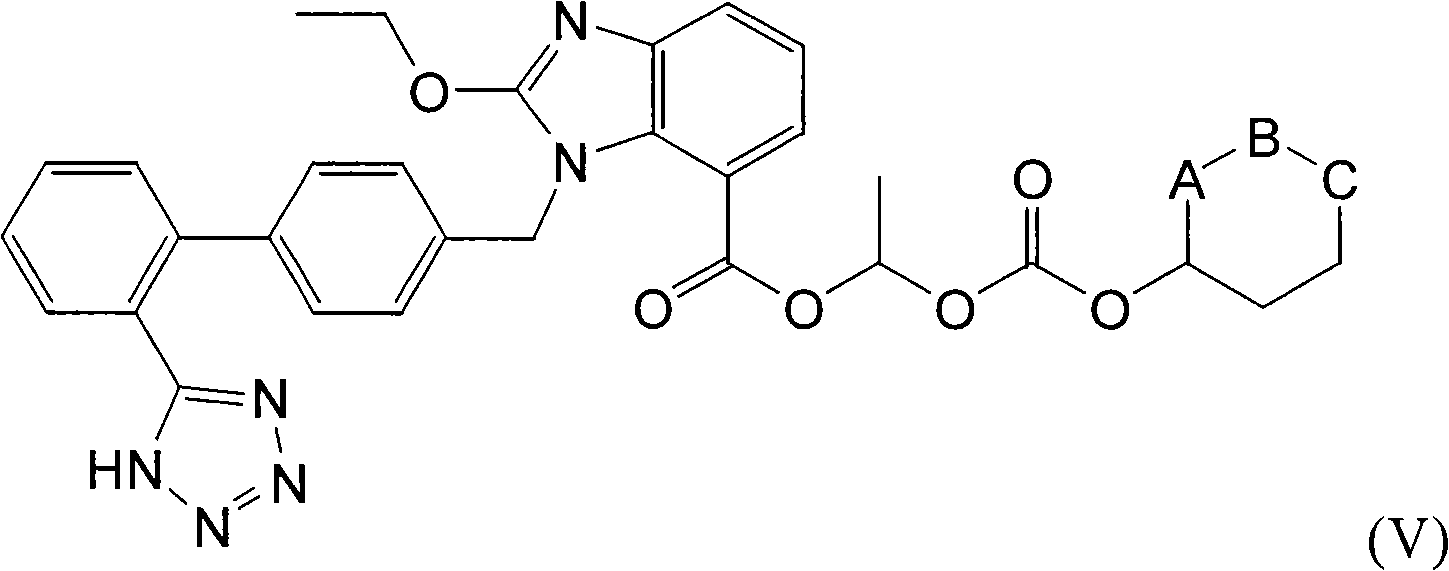
[0024] a) Esters of 1-((2'-cyano(1,1'-biphenyl)-4-yl)methyl)-2-ethoxy-1H-benzimidazole-7-carboxylate , preferably an alkyl ester thereof, converted into an ester, preferably a candesartan alkyl ester;
[0025] b) hydrolyzing the candesartan (alkyl) ester to obtain candesartan;
[0026] c) tritylating candesartan to obtain trityl candesartan;
[0027] d) esterification of trityl candesartan to obtain trityl candesartan cilexetil;
[0028] e) deprotecting trityl candesartan in the presence of Lewis acid to obtain candesartan cilexetil;
[0029] f) Crystallization of candesartan cilexetil from alcohol to obtain candesartan cilexeti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com