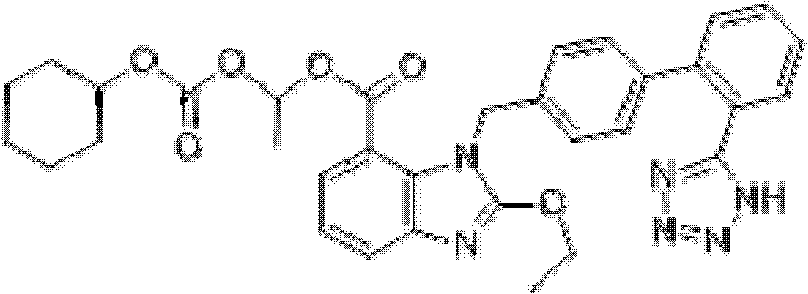
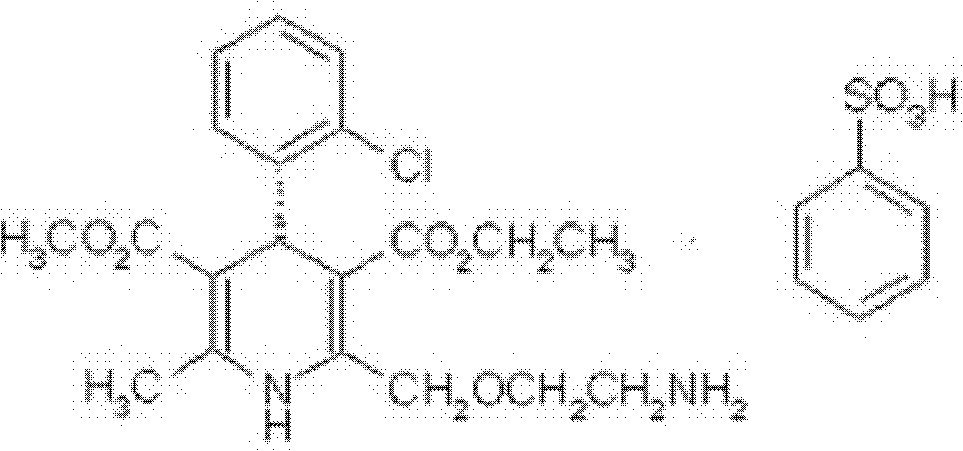
Brand new drug composition containing levamlodipine besylate and candesartan cilexetil and preparation method thereof
A kind of technology of levamlodipine besylate and candesartan cilexetil, which is applied in the new pharmaceutical composition containing levamlodipine and candesartan cilexetil and the field of preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] [embodiment 1] preparation of levamlodipine besylate crystal
[0075] 1) dissolving levamlodipine besylate in a mixed solvent of dichloromethane and ethanol to obtain a dichloromethane / ethanol solution of levamlodipine besylate;
[0076] 2) Add n-heptane dropwise to the dichloromethane / ethanol solution of levamlodipine besylate obtained in step 1) under an ultrasonic field until crystallization occurs;
[0077] 3) Turn off the ultrasonic field, let stand, filter, wash the filter cake with dichloromethane and ethanol respectively, and dry to obtain the levamlodipine besylate crystal.
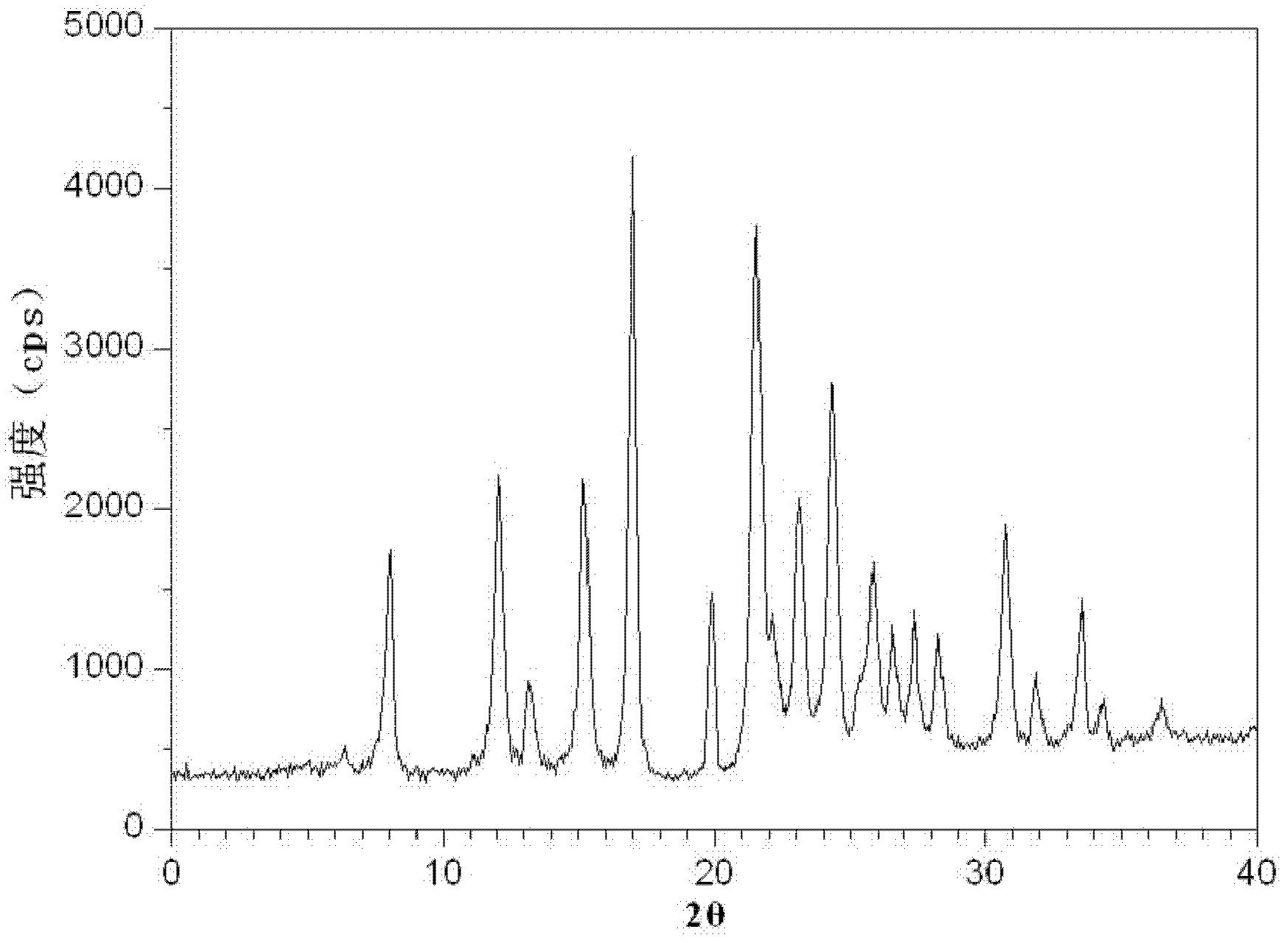
[0078] Gained levamlodipine besylate crystals use Cu-Kα rays to measure characteristic peaks in the X-ray powder diffraction pattern obtained at 2θ of 8.0°, 12.1°, 15.4°, 17.0°, 19.8°, 21.6°, 23.0° , 24.3°, 25.7°, 27.4°, 30.7° and 33.5° display, such as figure 1 shown.
[0079] Below is embodiment 2-9, and preparation method is with embodiment 1, and its concrete process parameter is sh...
Embodiment 1
[0085] [Formulation Example 1] Film-coated tablets of levamlodipine besylate and candesartan cilexetil
[0086] 1. Prescription
[0087] Tablet prescription:
[0088]
[0089]
[0090] Coating Solution Prescription:
[0091]
[0092] 2. Preparation process
[0093] (1) Candesartan cilexetil is passed through an 80-mesh sieve, for subsequent use;
[0094] (2) Microcrystalline cellulose, compressible starch, croscarmellose sodium, silicon dioxide and magnesium stearate were respectively baked at 60°C for 4 hours, passed through a 60-mesh sieve, and set aside;
[0095] (3) Take the above-mentioned standby candesartan cilexetil, microcrystalline cellulose, croscarmellose sodium, silicon dioxide and magnesium stearate according to the recipe quantity, and mix them uniformly by equal addition method to obtain mixed powder;
[0096] (4) Take by weighing the levamlodipine besylate crystal prepared in Example 1 of the prescription amount, mix with the mixed powder obtaine...
Embodiment 2
[0101] [Formulation Example 2] Film-coated tablets of levamlodipine besylate and candesartan cilexetil
[0102] 1. Prescription
[0103] Tablet prescription:
[0104]
[0105]
[0106] Coating Solution Prescription:
[0107]
[0108] 2. Preparation process: same as preparation example 1, the difference is that the levamlodipine besylate used is the levamlodipine besylate crystal prepared in Example 2, and step 2) is to bake at 80°C 2 hours, through 80 mesh sieves.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com