Candesartan cilexetil composition
A technology of candesartan cilexetil and its composition, which is applied in the field of candesartan cilexetil granules and its composition, and can solve the problems of small particle size and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
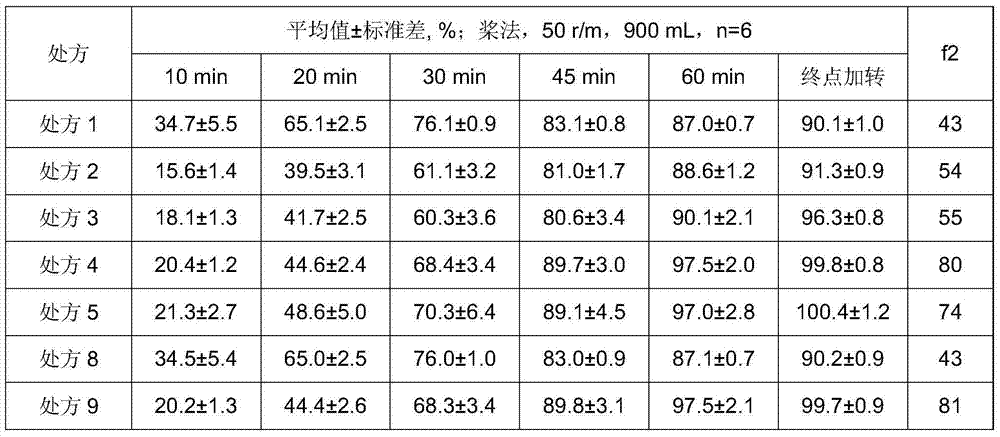
[0013] The second aspect provides candesa whose particle size (D90) corresponding to the cumulative particle size distribution number reaches 90% is less than 16 μm and the particle size (D90) corresponding to the cumulative particle size distribution number reaches 90% is higher than 11 μm. A method for preparing tansylate granules, which comprises: heating candesartan cilexetil to reflux and dissolving it in a solvent containing acetone, controlling the stirring speed to be V1, adding water; Removal of the solvent afforded the product.
[0014] The acetone-containing solvent may or may not contain water. In some embodiments, the mass ratio of acetone to water in the acetone-containing solvent is 10:1-15:1. In some embodiments, the mass ratio of acetone to water in the acetone-containing solvent is 12:1-15:1.
[0015] The mass ratio of acetone to candesartan cilexetil is 3:1-5:1.
[0016] When water is added, the mass ratio of the added water to the candesartan cilexetil i...
specific Embodiment approach
[0053] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0054] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0055] In the present invention, min means minute, μm means micron, f2 means similarity factor, r / m means revolution / minute, mL means milliliter, kg means kilogram, mg means milligram, and parts refer to parts by weight.
Embodiment 1
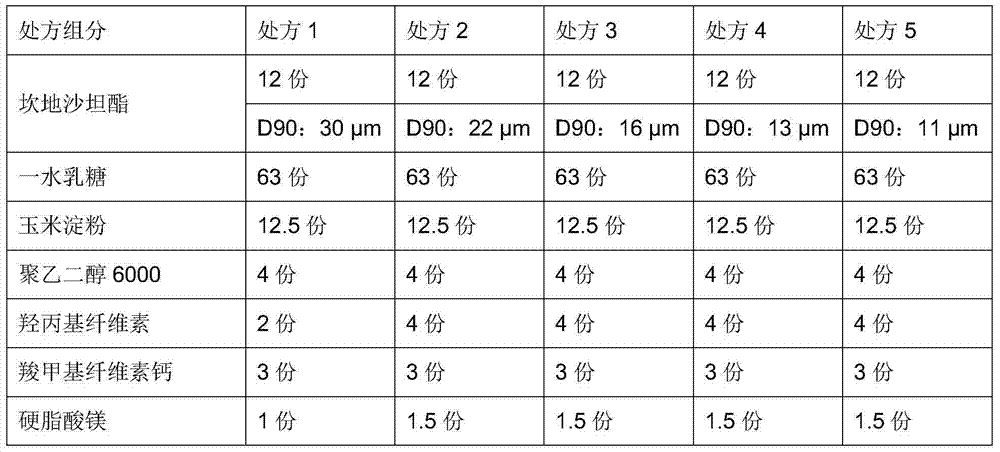
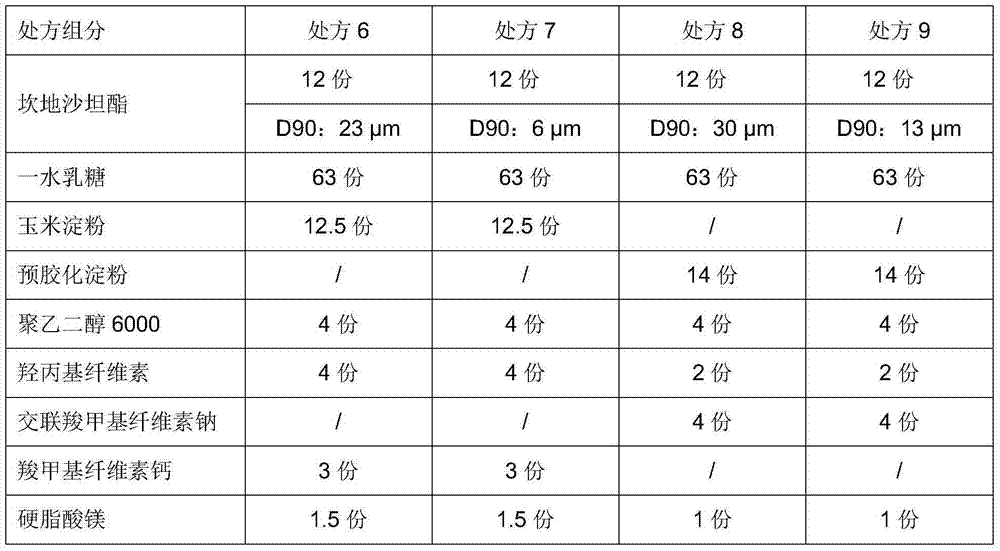
[0057] prescription:
[0058]
[0059]
[0060] Note: " / " means no such component.
[0061] Preparation:
[0062] Candesartan cilexetil, lactose monohydrate, corn starch (if any), pregelatinized starch (if any), hydroxypropyl cellulose, croscarmellose sodium (if any) weighed according to the prescription ), add water and mix evenly; then add polyethylene glycol, carboxymethylcellulose calcium (if any), and magnesium stearate; sieve; dry at 50-60°C, sieve for granulation, and compress into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com