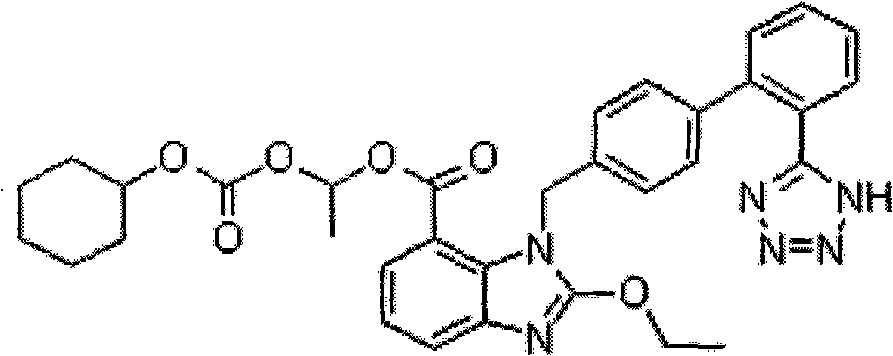
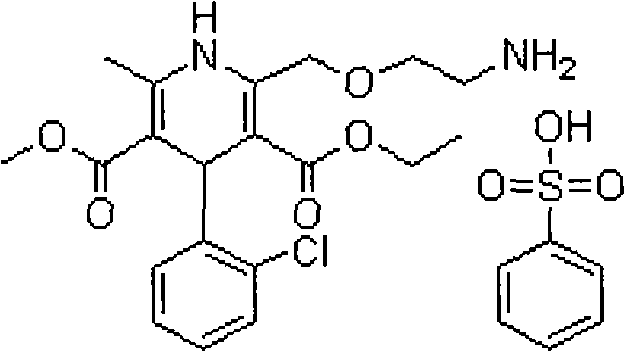
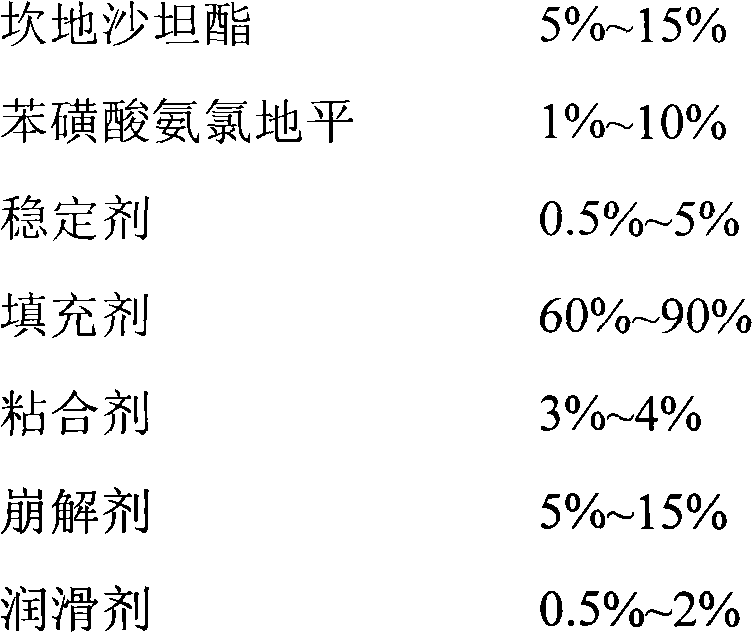
Oral tablet containing candesartan cilexetil and benzene sulfonate amlodipine and preparation method for oral tablet
A technology of amlodipine besylate and candesartan cilexetil, which is applied in the field of medicine, can solve problems such as one-sided unqualified, reduced dissolution characteristics of candesartan, and reduced disintegration of solid preparations, so as to achieve rapid dissolution and avoid Sticking phenomenon, avoiding disintegration and reducing the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046]Three prescriptions used copovidone at different ratios, among which: the ratio of copovidone used in prescription 1 was 2.0%; the ratio of copovidone used in prescription 2 was 3.0%; the ratio of copovidone used in prescription 3 was 4.0% %, the proportion of copovidone used in prescription 4 was 6.0%.
[0047] The particle size of candesartan cilexetil is D (V,0.9) =41μm, the particle size of amlodipine besylate is D (V,0.9) = 53 μm, absolute ethanol is removed during production.
[0048] The preparation method is as follows: dissolve the prescription amount of glycerin in an appropriate amount of absolute ethanol to make an adhesive for use; mix candesartan cilexetil, amlodipine besylate and other granulation auxiliary materials in a granulation pot to obtain the mixture A: Add the ethanol solution binder of glycerin into the granulation pot and granulate together with mixture A to obtain wet granules, dry the wet granules to obtain dry granules; mix dry...
Embodiment 2
[0061] The particle size of candesartan cilexetil is D (V,0.9) =22μm, the particle size of amlodipine besylate is D (V,0.9) =93 μm, absolute ethanol is removed during production.
[0062] The preparation method is as follows: dissolve the prescription amount of glycerin in an appropriate amount of absolute ethanol to make an adhesive for use; mix candesartan cilexetil, amlodipine besylate and other granulation auxiliary materials in a granulation pot to obtain the mixture A: Add the ethanol solution binder of glycerin into the granulation pot and granulate together with mixture A to obtain wet granules, dry the wet granules to obtain dry granules; mix dry granules with lubricant to obtain mixed granules; use rotary tablet press for mixed granules Carry out tabletting, tablet weight 130mg, make 5000 tablets.
[0063] According to dissolution assay method (Chinese Pharmacopoeia 2010 edition appendix XC second method), use the 900ml pH6.5 phosphate buffer that adds 0.35%...
Embodiment 3
[0068] The particle size of candesartan cilexetil is D (V,0.9) =57μm, the particle size of amlodipine besylate is D (V,0.9) = 33 μm, absolute ethanol is removed during production.
[0069] The preparation method is as follows: dissolve the prescription amount of glycerin in an appropriate amount of absolute ethanol to make an adhesive for use; mix candesartan cilexetil, amlodipine besylate and other granulation auxiliary materials in a granulation pot to obtain the mixture A: Add the ethanol solution binder of glycerin into the granulation pot and granulate together with mixture A to obtain wet granules, dry the wet granules to obtain dry granules; mix dry granules with lubricant to obtain mixed granules; use rotary tablet press for mixed granules Carry out tabletting, tablet weight 130mg, make 5000 tablets.
[0070] According to dissolution assay method (Chinese Pharmacopoeia 2010 edition appendix XC second method), use the 900ml pH6.5 phosphate buffer that adds 0.35...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com