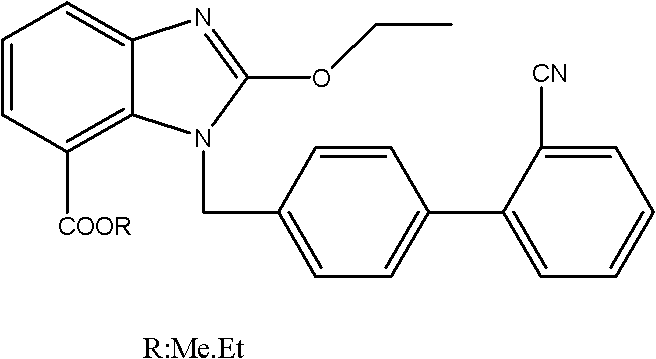
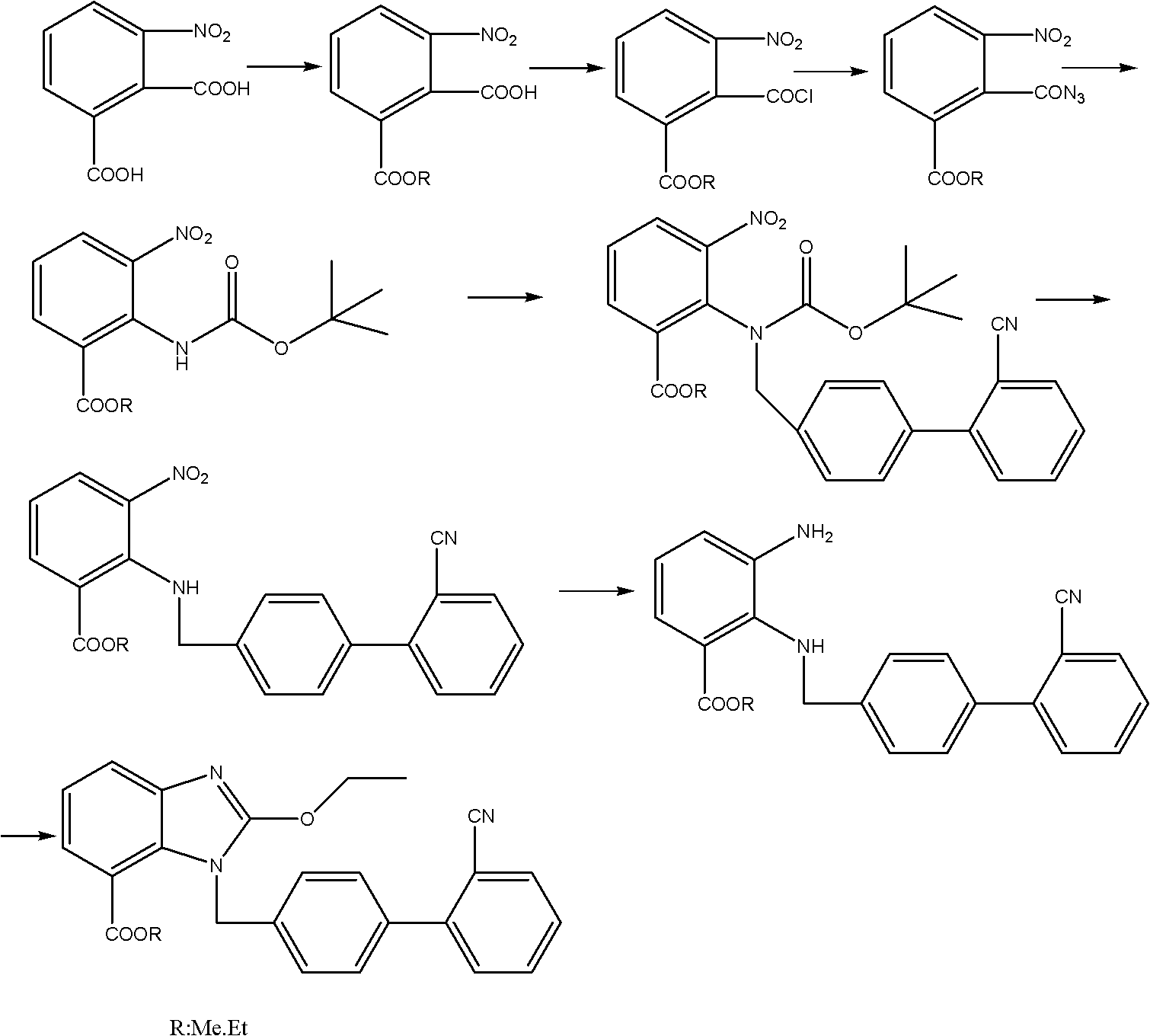
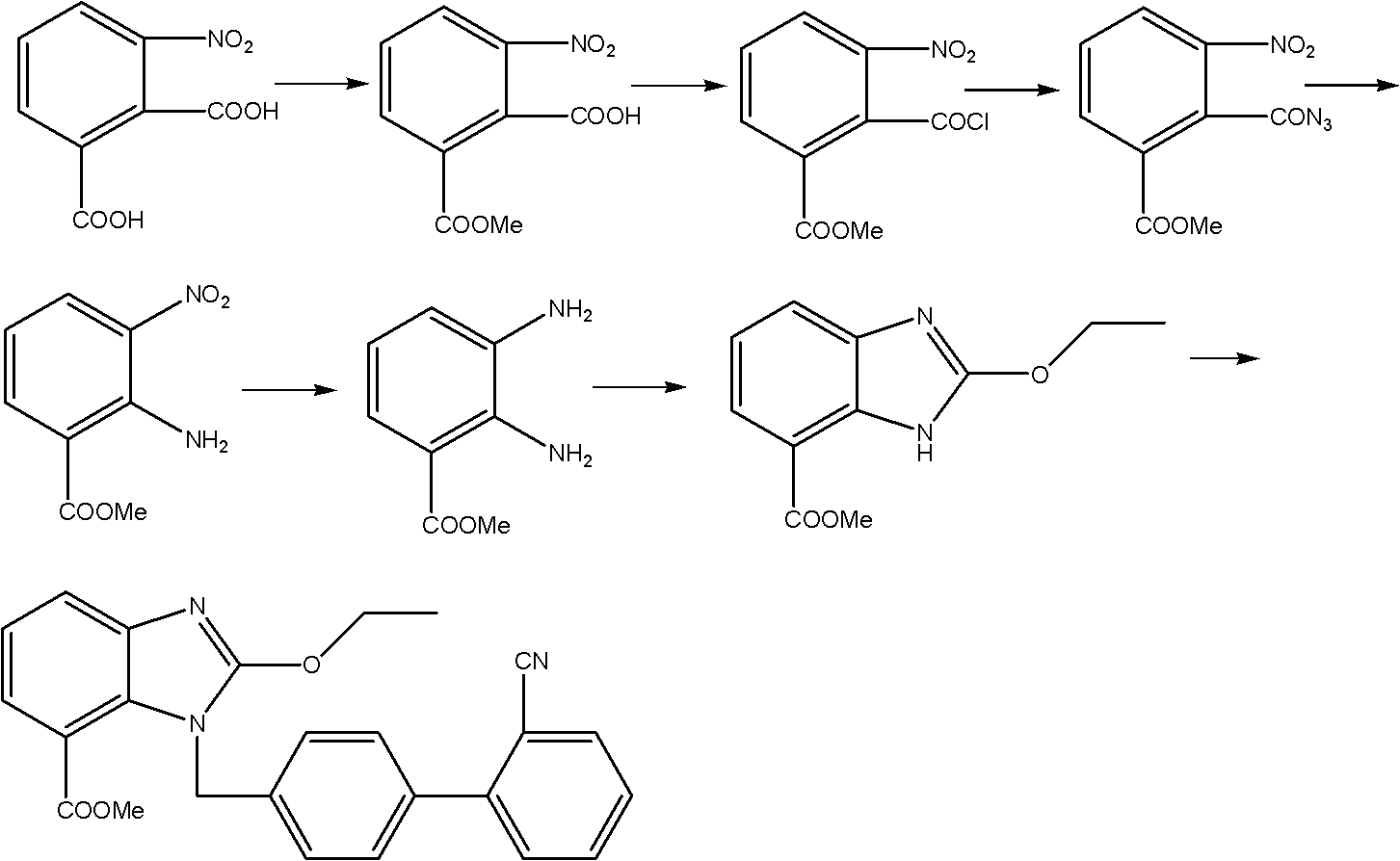
Method for preparing candesartan ring compound
A technology of candesartan and cyclic compound, which is applied in the field of preparation of candesartan cyclic compound, can solve the problems of high consumption and high final cost of candesartan cyclic compound, and achieve low cost The effect of minimization and consumption minimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Step 1) the preparation of ethyl 3-nitro-2-ethoxycarboxamidobenzoate: 1. monoesterification: get dehydrated alcohol 400g, 3-nitrophthalic acid 90g and the vitriol oil 90g mix, control Temperature 70°C, heat and reflux for 12 hours, distill off ethanol under reduced pressure, add 1000g of chloroform, stir, extract and separate layers to obtain a monoesterified chloroform solution; To complete, then add 4g DMF and 45g thionyl chloride, control the temperature at 60°C, reflux for 5 hours, distill off 500g of chloroform to obtain a chloroform solution of acid chloride; ③ azidation: add 50g of DMF to the chloroform solution of acid chloride , 30g of sodium azide, react at room temperature 25°C for 3 hours, add 500g of chloroform, 500g of water, extract and separate layers, and obtain the chloroform solution of acyl azide; Add 200g of Yuanming powder for drying and dehydration, filter, add 300g of absolute ethanol to the filtrate, slowly heat to reflux, control the temperatur...
Embodiment 2
[0053] Step 1) the preparation of 3-nitro-2-ethoxycarboxamidoethyl benzoate: 1. monoesterification: get dehydrated alcohol 1800g, 3-nitrophthalic acid 450g and the concentrated sulfuric acid 485g mix, control Temperature 70°C, heat and reflux for 17 hours, distill off ethanol under reduced pressure, add 4500g of chloroform, stir, extract and separate layers to obtain a monoesterified chloroform solution; To complete, then add 20g DMF and 250g thionyl chloride, control the temperature at 60°C, reflux for 5 hours, distill off 2600g of chloroform to obtain a chloroform solution of acid chloride; ③ azidation: add 260g of DMF to the chloroform solution of acid chloride , 150g of sodium azide, react at room temperature 25°C for 3 hours, add 2500g of chloroform, 2600g of water, extract and separate layers, and obtain the chloroform solution of acyl azide; Add 1000g of Yuanming powder for drying and dehydration, filter, add 1500g of absolute ethanol to the filtrate, slowly heat to ref...
Embodiment 3
[0058] Step 1) Preparation of 3-nitro-2-ethoxycarboxamidoethyl benzoate: 1. monoesterification: dehydrated alcohol 40Kg, 3-nitrophthalic acid 9Kg, concentrated sulfuric acid 9Kg, heat reflux reaction 18 Hours, evaporate ethanol under reduced pressure, add 100Kg of chloroform, stir, extract, and separate layers to obtain a monoesterified chloroform solution; 4.5Kg of thionyl chloride, reflux reaction for 5 hours, distilled off 50Kg of chloroform to obtain a chloroform solution of acid chloride; ③Azidation: Add 5.4Kg of DMF and 3Kg of sodium azide to the chloroform solution of acid chloride, and react at room temperature for 3 4 hours, add 50Kg of chloroform, 50Kg of water, extract and separate layers to obtain the chloroform solution of acyl azide; ④ rearrangement esterification: add 20Kg of chloroform solution of acyl azide, dry and dehydrate, filter, add the filtrate 30Kg of absolute ethanol, slowly heated to reflux, reflux for 8 hours, distilled off chloroform and ethanol, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com