Process for preparing candesartan cilexetil C-form crystal
A technology for candesartan cilexetil and crystals is applied in the field of preparation of organic compound crystals, and can solve the problems of low one-time yield, small difference in boiling point, large loss of raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
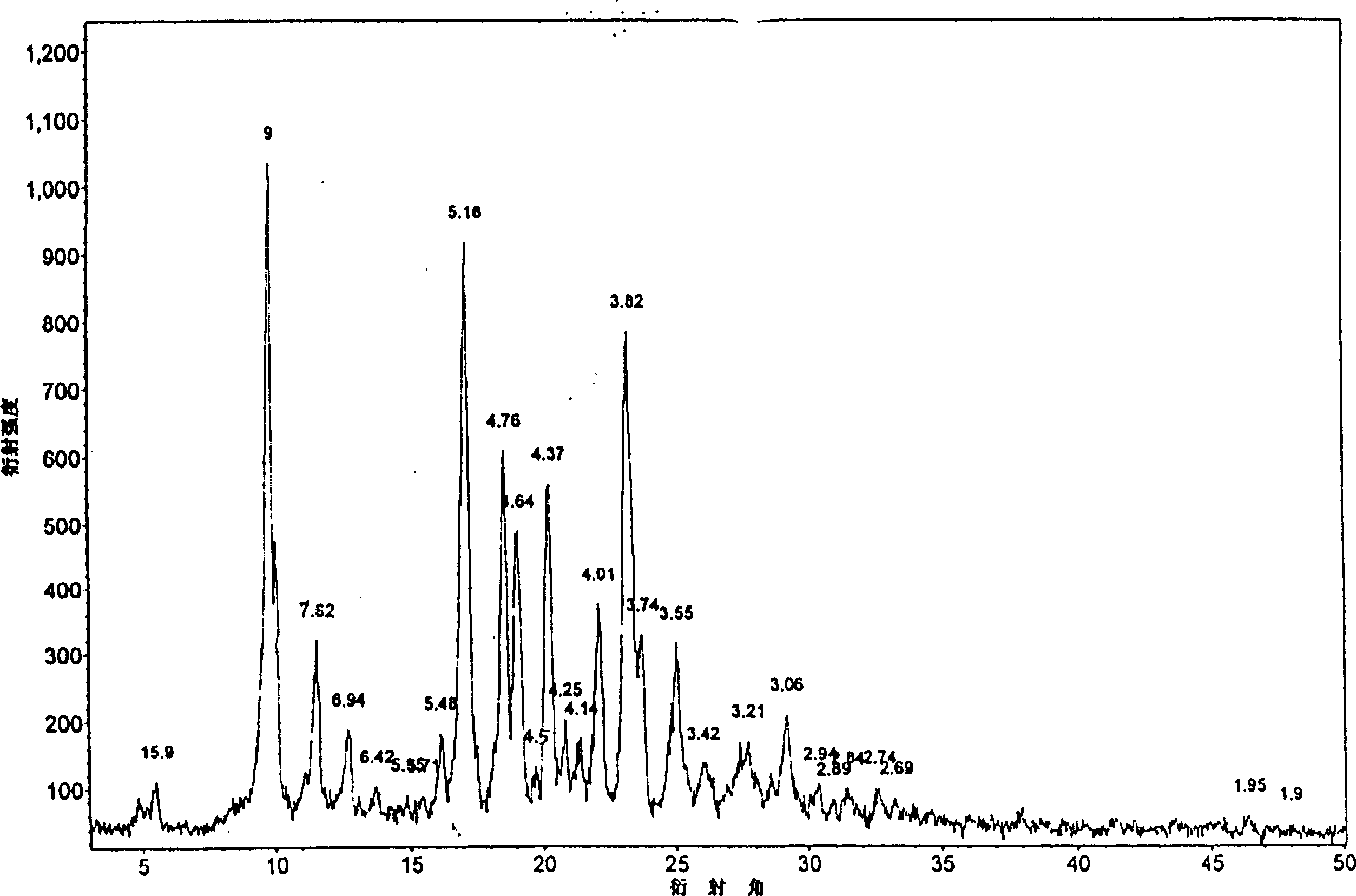
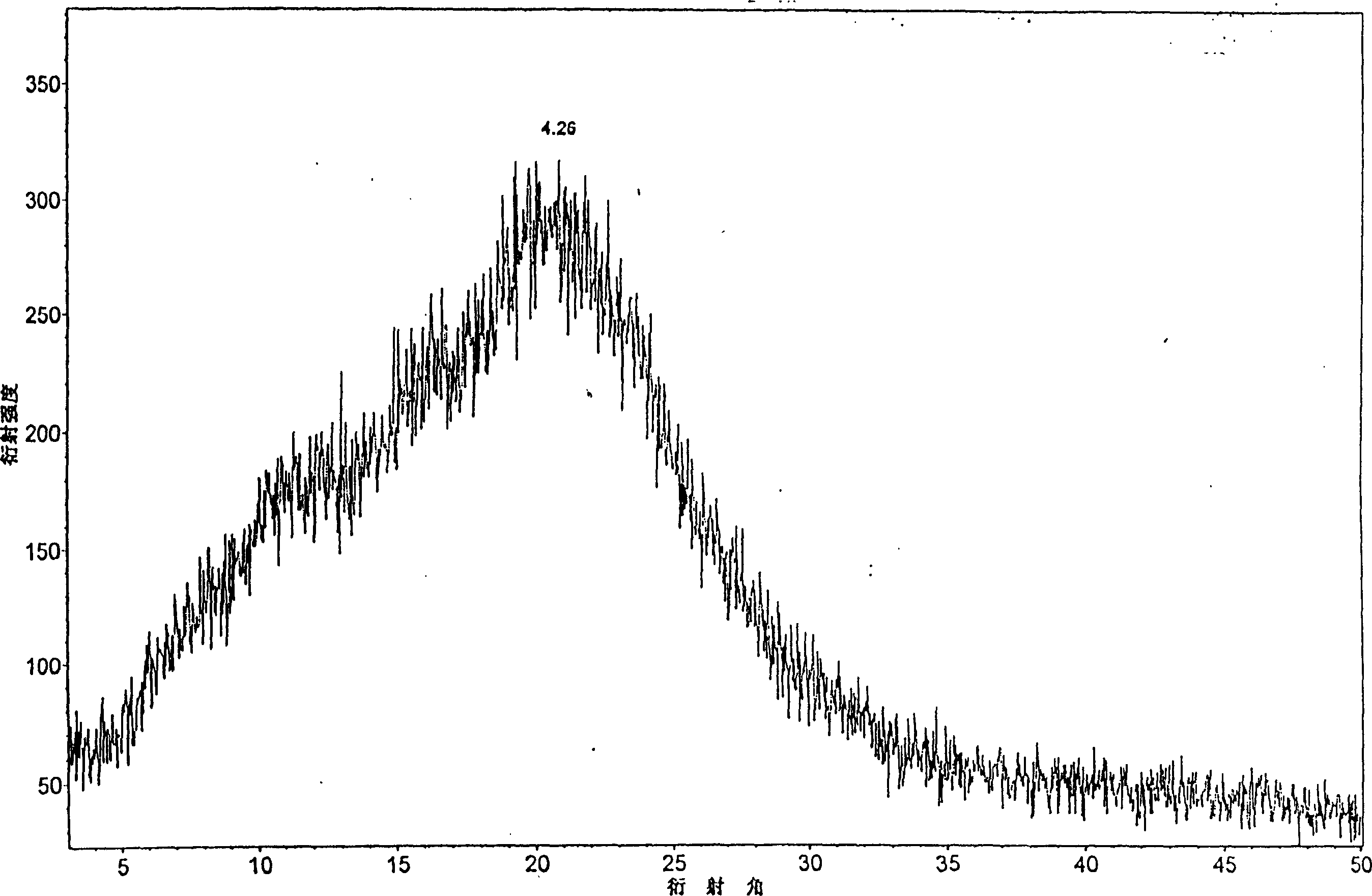
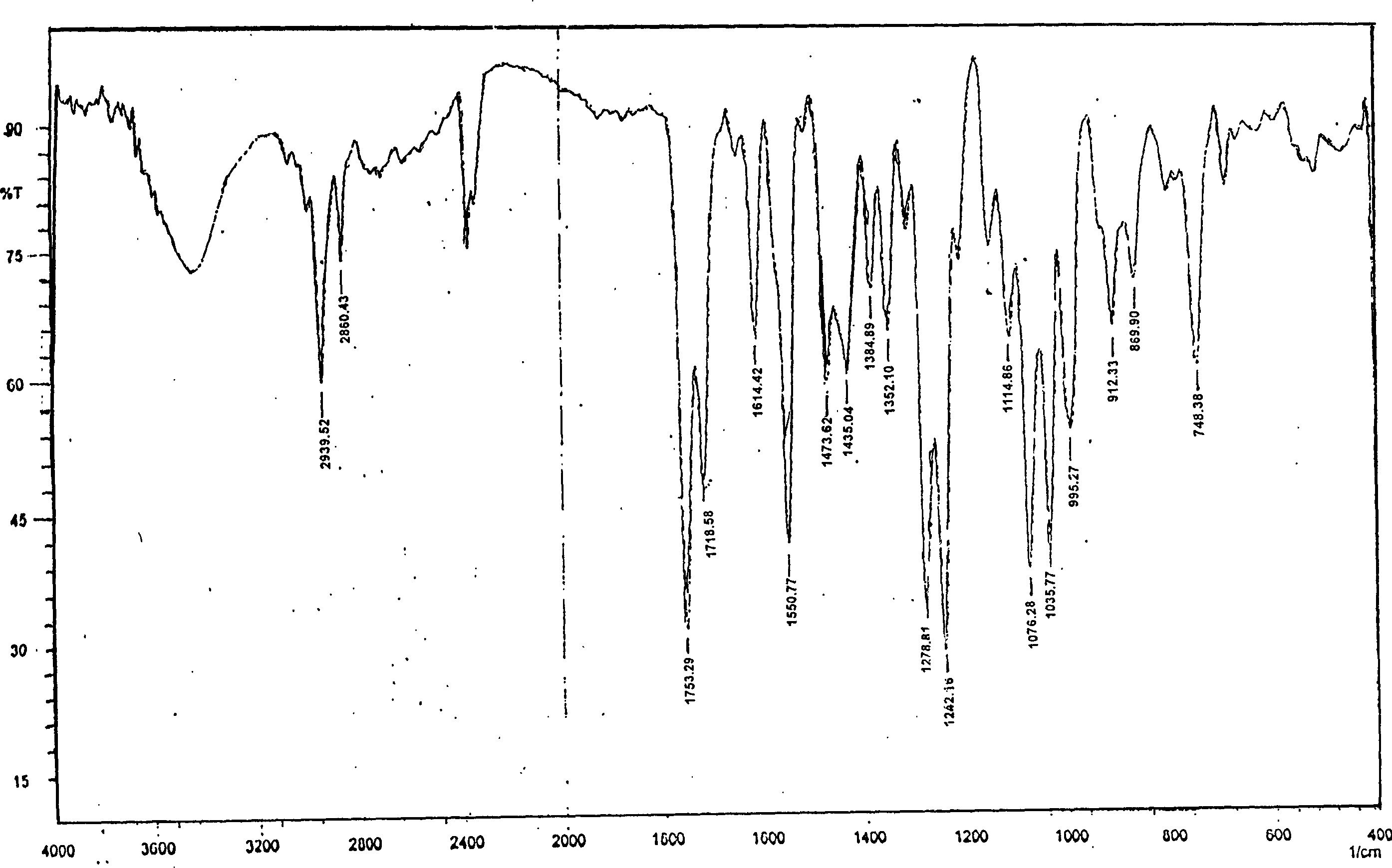
[0020] Add 10g of candesartan cilexetil non-C-type crystal powder into 20ml of dimethylformamide aqueous solution, stir at room temperature to dissolve, then add 100ml of water dropwise to the organic solution under continuous stirring, stir for 1 to 5 hours, and precipitate crystals , suction filtration, and vacuum drying to obtain 10 g of the product. The HPLC method measures its content to be 99.4%, and melting point is 164 ℃~166 ℃, and the X-ray diffraction pattern of gained crystal is as follows: figure 1 Shown; The infrared spectrogram of the obtained crystal is shown in image 3 shown. The corresponding X-ray diffraction pattern and the infrared spectrogram of the candesartan cilexetil C-type crystal reported by aforementioned CN1058966C are as follows respectively Figure 4 and Figure 5 shown. The X-ray diffraction contrast pattern of the non-C-type crystal powder of candesartan cilexetil as raw material used in the present invention is as follows figure 2 shown...
Embodiment 2
[0022] Add 10 g of candesartan cilexetil non-C-form crystal into 20 ml of diethylene glycol dimethyl ether aqueous solution, stir and dissolve at room temperature, add 150 ml of water dropwise under stirring, continue stirring for 1 to 5 hours and lower the temperature to 5°C to 10°C, Crystals were precipitated, filtered by suction, and dried in vacuo to obtain 10 g of the product. The HPLC method determines its content to be 99.5%, and its melting point is 160°C to 163°C.
Embodiment 3
[0024] Add 10 g of candesartan cilexetil non-C-form crystal into 30 ml of acetonitrile aqueous solution, stir and dissolve at room temperature, add 350 ml of water dropwise under continuous stirring and lower the temperature to below 40°C, keep stirring for 1 to 5 hours, precipitate crystals, filter with suction, Drying in vacuo yielded 9.8 g of product. As determined by HPLC method, its content is 99.2%, and its melting point is 158°C-163°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com