Preparation method of candesartan cilexetil
A technology of candesartan cilexetil and candesartan acid, applied in the field of medicine, can solve the problems of poor product quality, cumbersome synthesis process, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
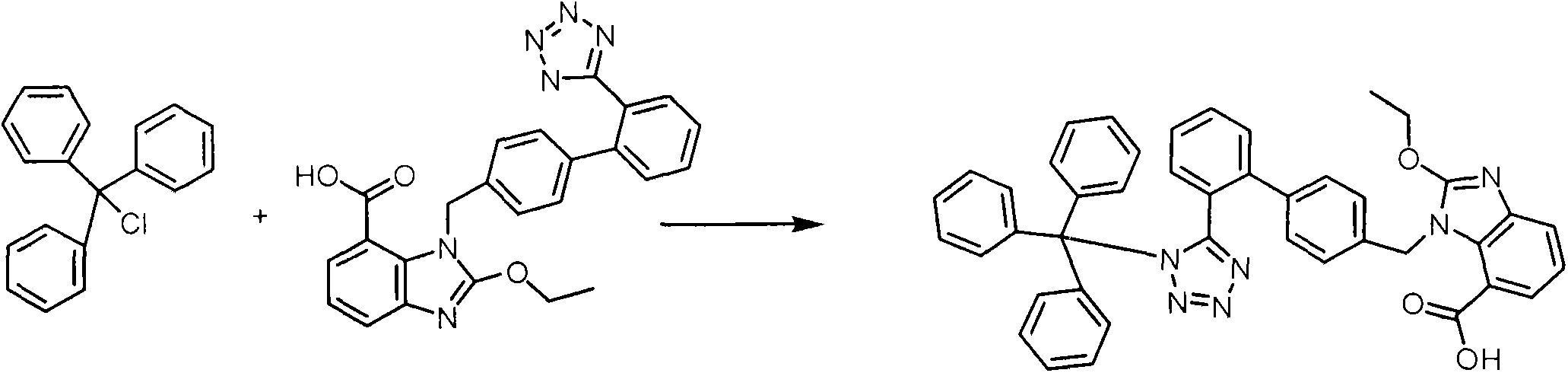
[0030] The first step, add 130 kilograms of N in the 500L reactor, N dimethylacetamide, drop into 22 kilograms of candesartan acid and 17.3 kilograms of anhydrous potassium carbonate, 16.7 kilograms of trityl chloride, tetrabutyl bromide under stirring 0.2 kg of ammonium chloride, stirred for 10 minutes, started to heat up to 63±3°C, stirred and reacted, and the remaining amount of candesartan acid in the reaction solution was detected by HPLC <2.0%. The reaction solution was directly reserved for feeding in the next step.
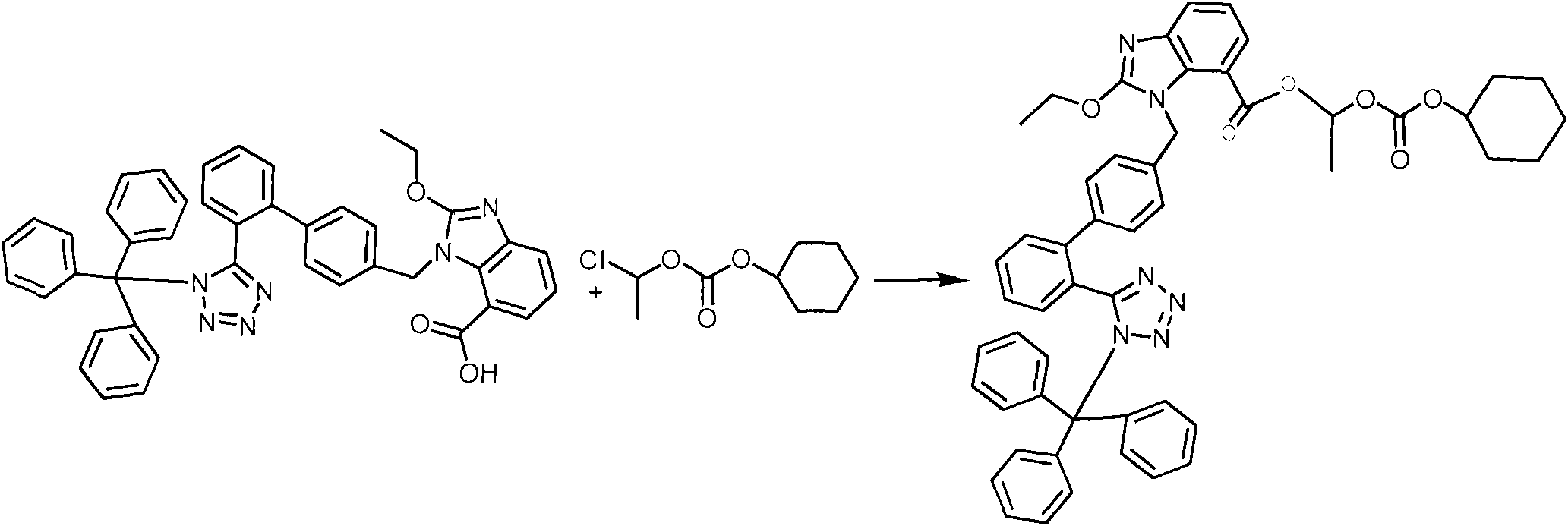
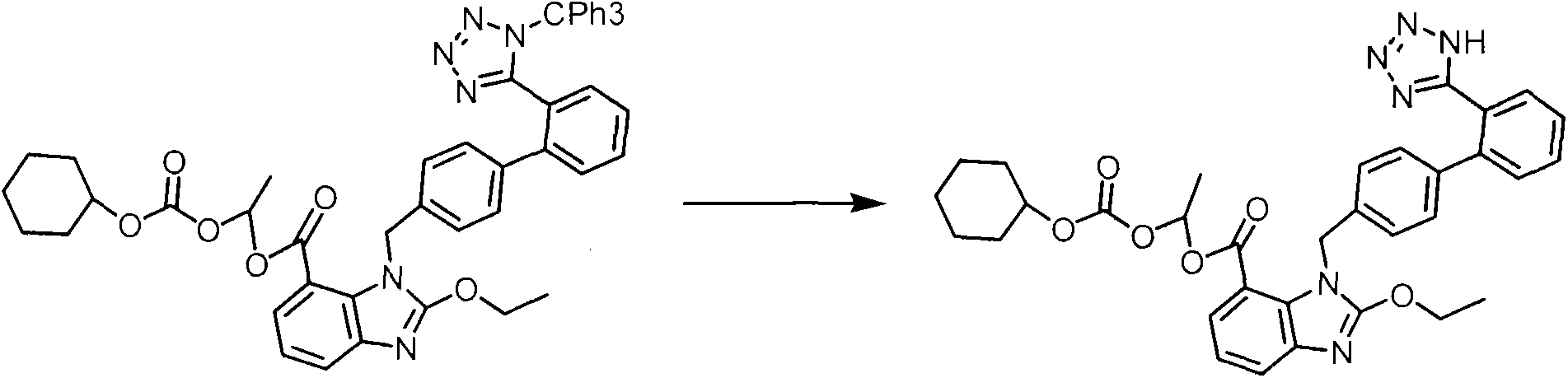
[0031] In the second step, the temperature of the reaction solution in the first step is lowered to 55°C, 12.4 kg of 1-chloroethylcyclohexyl carbonate is added, and the temperature is controlled at 55-60°C for reaction. The raw materials of the reaction solution are completely reacted by HPLC, and the temperature of the reaction solution is cooled to 20°C. Under stirring, inject the reaction solution into 400 kg of ice water at a slow speed, precipitate so...
example 2
[0035] In the first step, add 260 kilograms of N,N-dimethylformamide into a 500L reactor, and add 44 kilograms of candesartan acid and 25.3 kilograms of triethylamine, 33.5 kilograms of trityl chloride, and triethyl chloride under stirring. 0.5 kg of benzyl ammonium, after stirring evenly, start to heat up to 80° C., stirring and reacting, HPLC detects that the remaining amount of candesartan acid in the reaction solution is <2.0%. The reaction solution was directly reserved for feeding in the next step.
[0036] In the second step, the temperature of the reaction solution in the first step was lowered to 55°C, 24.8 kg of 1-chloroethylcyclohexyl carbonate was added, and the temperature was controlled at 50-55°C to react. The raw materials of the reaction liquid were completely reacted by HPLC, and the temperature of the reaction liquid was lowered to 20°C. Under stirring, inject the reaction liquid into 800 kg of ice water at a slow speed, precipitate solids, filter, wash the ...
example 3
[0040]In the first step, add 232 kilograms of dimethyl sulfoxide to a 500L reactor, add 33 kilograms of candesartan acid and 30.0 kilograms of potassium bicarbonate, 25.1 kilograms of trityl chloride, and 0.3 kilograms of potassium iodide under stirring, and start The temperature was raised to 88±2°C, and the reaction was stirred, and the remaining amount of candesartan acid in the reaction liquid was detected by HPLC to be <2.0%. The reaction solution is fed directly to the next step.
[0041] In the second step, the temperature of the reaction liquid in the first step is lowered to 55°C, 18.6 kg of 1-chloroethylcyclohexyl carbonate is added, and the temperature is controlled at 55-60°C for 6 hours. The raw materials of the reaction liquid are completely reacted by HPLC, and the temperature of the reaction liquid is lowered to 20°C. ℃, under stirring, slowly inject the reaction solution into 600 kg of ice water, precipitate solids, filter, wash the filter cake with water, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com