High performance liquid chromatography for analyzing candesartan cilexetil
A technology of high performance liquid chromatography and candesartan cilexetil, applied in the field of medicine, can solve the problem that the actual degradation products of candesartan cilexetil are not controlled
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Instruments and Conditions:
[0031] High performance liquid chromatography: WATERS 2996, PDA detector
[0032] Chromatographic column: Agilent ZORBAX SB-C8, 4.6×100mm, 3.5μm; Buffer: Weigh 1.4g of potassium dihydrogen phosphate into 1L of water, adjust the pH to 2.2 with phosphoric acid, filter, as a buffer; mobile phase: phosphoric acid in acetonitrile Buffer; flow rate: 1.2mL / min; detection wavelength: 230nm; column temperature: 40°C; injection volume: 20μL.
[0033] Experimental steps:
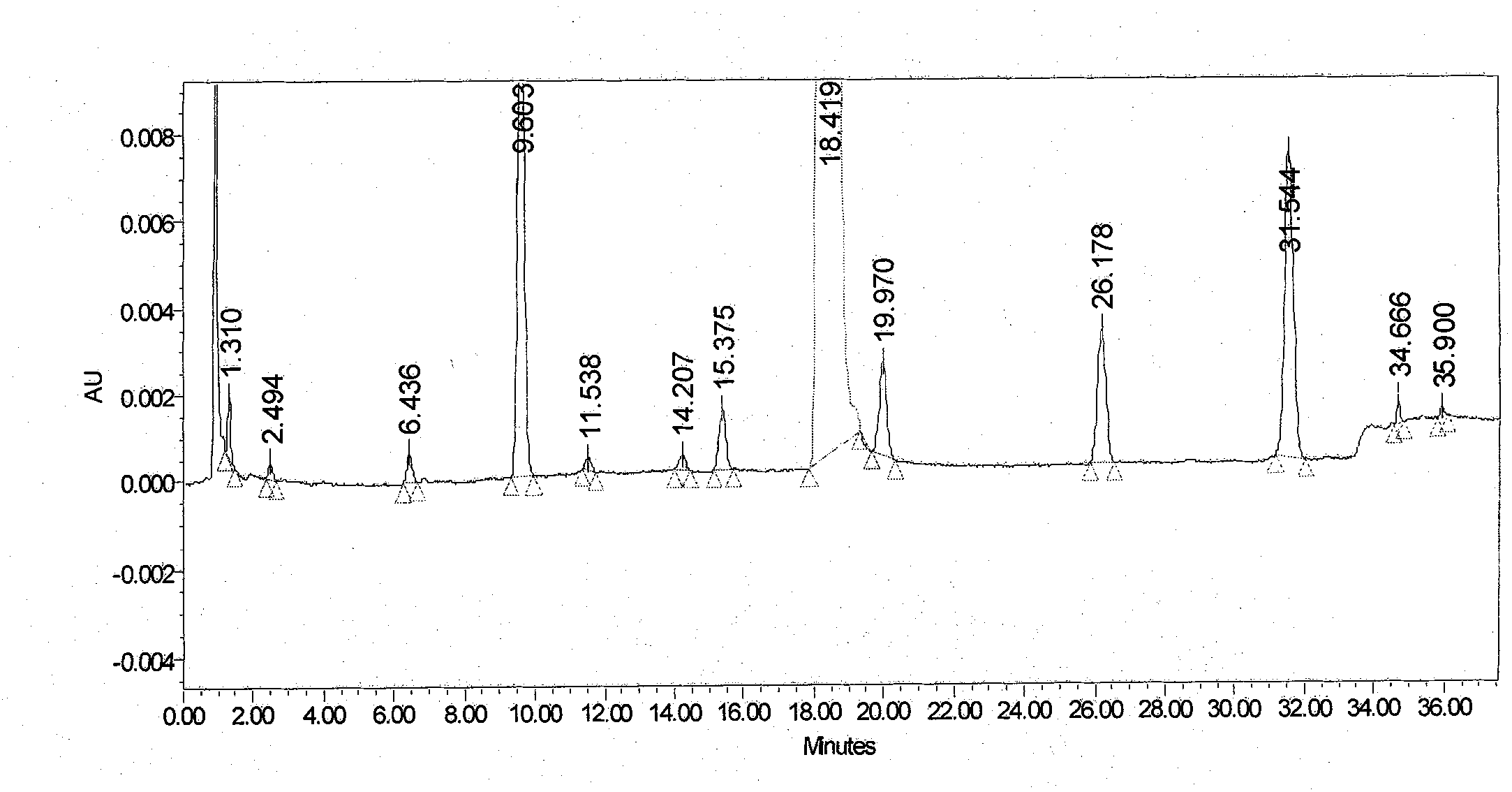
[0034] (1) Take an appropriate amount of candesartan cilexetil tablet 2 mg accelerated March sample powder, dissolve it with acetonitrile: water = 3: 2 ultrasonically for 15 minutes, make a sample solution containing candesartan cilexetil 0.5 mg / mL, centrifuge, and take The supernatant was used as the sample solution.
[0035] (2) Take the above sample solution, use acetonitrile-phosphate buffer solution as the organic phase, inject 20 μL of the sample, and carry out gradient elu...
Embodiment 2
[0038] Instruments and Conditions:
[0039] High performance liquid chromatography: WATERS 2996, PDA detector
[0040] Chromatographic column: Agilent ZORBAX SB-C8, 4.6×100mm, 3.5μm; Buffer: Weigh 1.4g of ammonium dihydrogen phosphate into 1L of water, adjust the pH to 2.4 with phosphoric acid, filter, as a buffer; mobile phase: phosphoric acid in acetonitrile Buffer; flow rate: 1.0mL / min; detection wavelength: 230nm; column temperature: 30°C; injection volume: 20μL
[0041] Experimental steps:
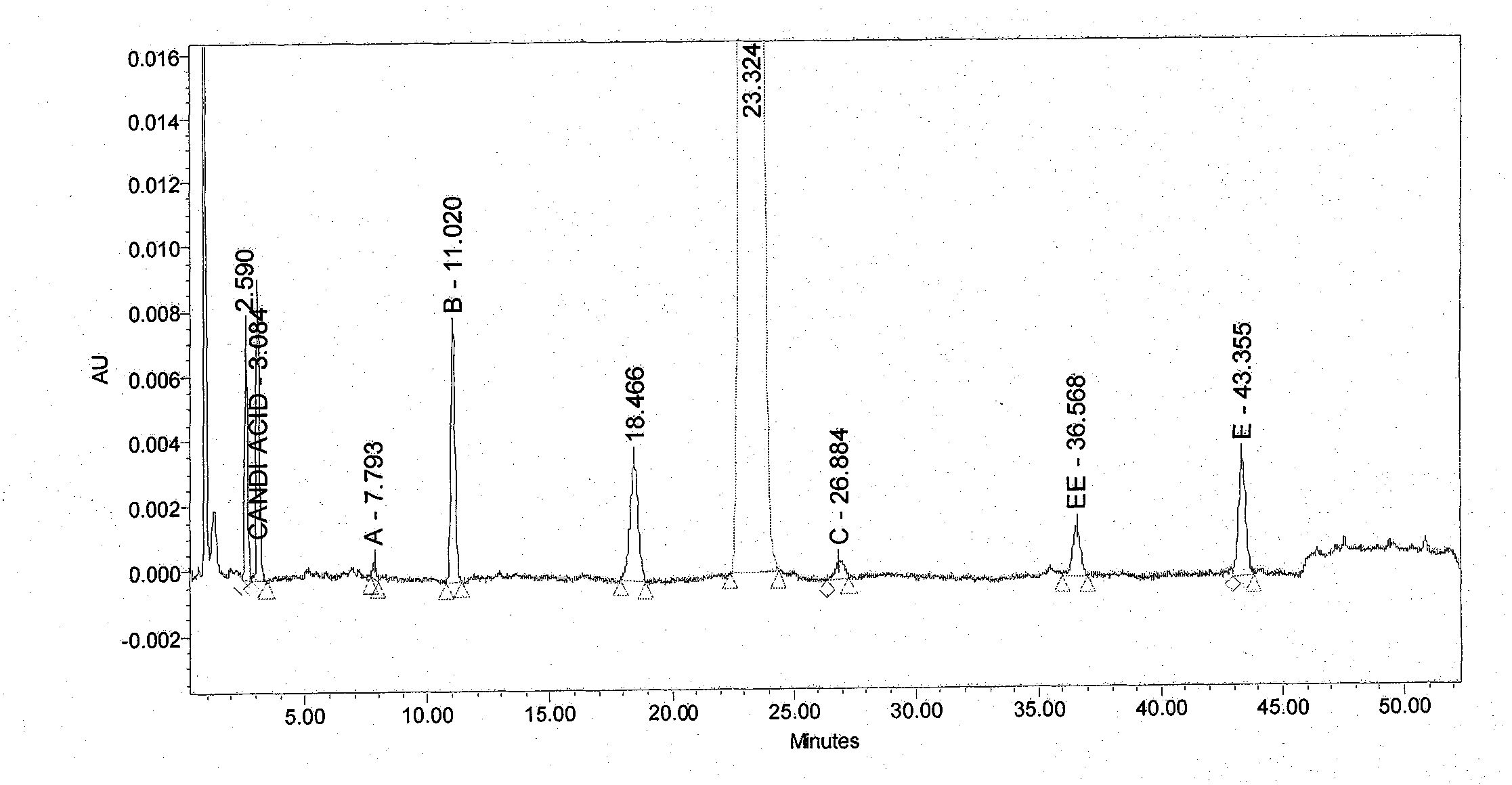
[0042] (1) Take an appropriate amount of candesartan cilexetil tablet 2 mg accelerated March sample powder, dissolve it ultrasonically with acetonitrile: water = 3: 2, make a sample solution containing candesartan cilexetil 0.5 mg / mL, filter it, and use it as a sample solution.
[0043] (2) Take the above sample solution, use acetonitrile phosphate buffer as the organic phase, inject 20 μL of the sample, and perform gradient elution according to the following conditions. The main gra...
Embodiment 3
[0046] Instruments and Conditions:
[0047] High performance liquid chromatography: WATERS 2996, PDA detector
[0048] Chromatographic column: Agilent ZORBAX SB-C8, 4.6×100mm, 3.5μm; Buffer: Weigh 1.4g of ammonium dihydrogen phosphate into 1L of water, adjust the pH to 2.4 with phosphoric acid, filter, as a buffer; mobile phase: phosphoric acid in acetonitrile Buffer; flow rate: 1.0mL / min; detection wavelength: 230nm; column temperature: 30°C; injection volume: 20μL
[0049] Experimental steps:
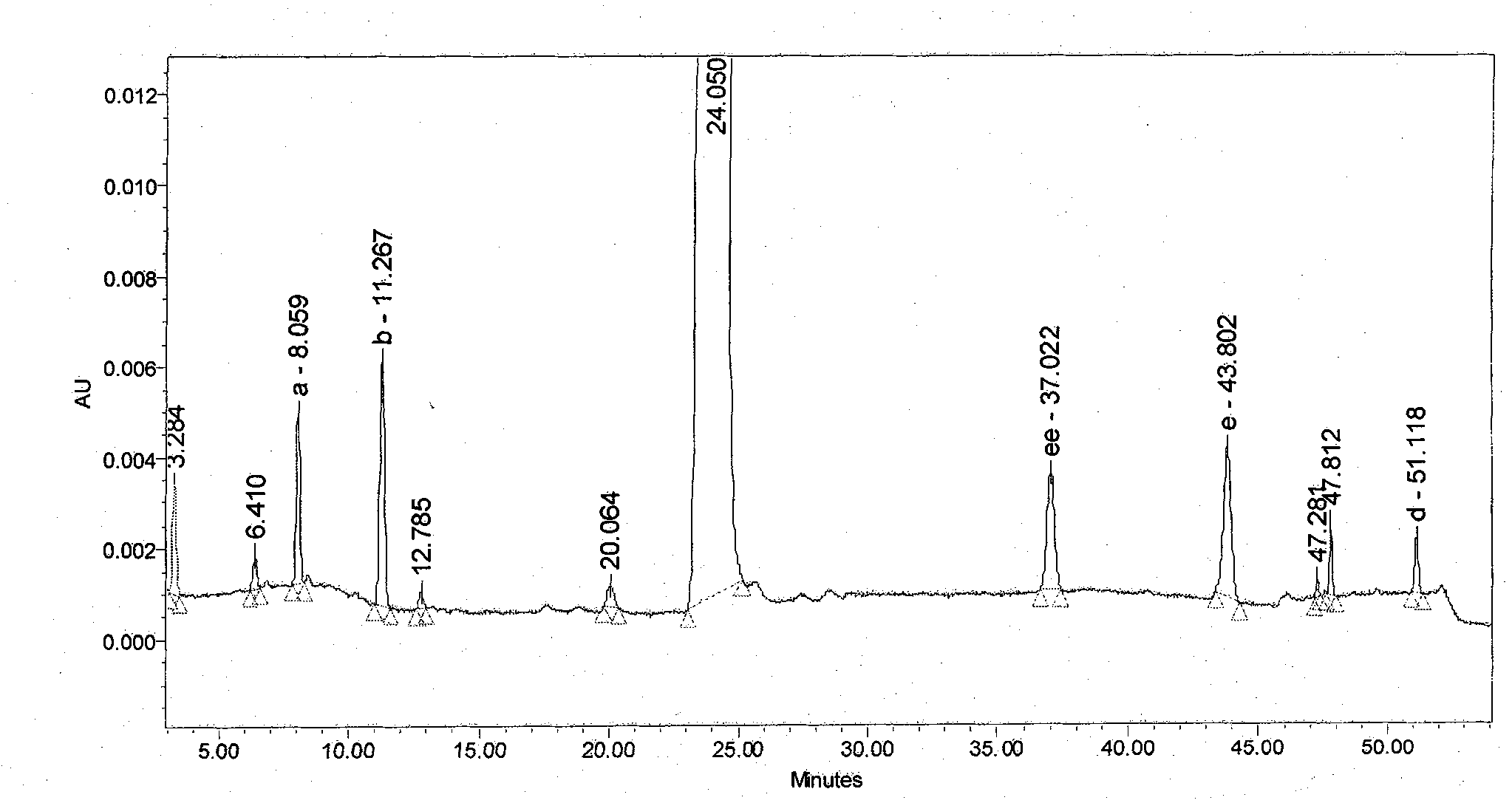
[0050] (1) Alkali destruction: Take an appropriate amount of candesartan cilexetil tablet 2mg specification sample powder into a 10mL volumetric flask, add 2mL of 1N sodium hydroxide solution, leave it at room temperature for half an hour, add 1N hydrochloric acid to neutralize, dilute to volume with acetonitrile, filter, Acts as a base to destroy the sample.
[0051] (2) Acid destruction: Take an appropriate amount of candesartan cilexetil tablet 2mg sample powder into a 10mL volu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com