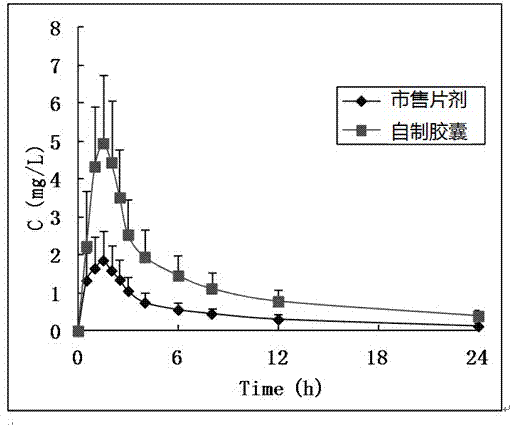
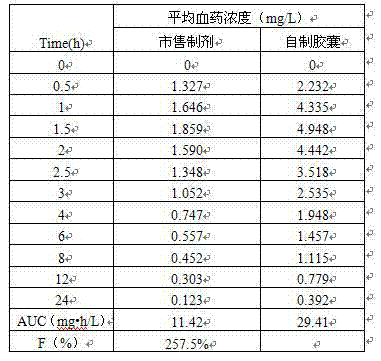
Preparation method of compound preparation for treating high blood pressure
A high blood pressure and compound technology, which is applied in cardiovascular system diseases, capsule delivery, active ingredients of heterocyclic compounds, etc., can solve the problems of low oral bioavailability of valsartan, complicated production processes, and low oral bioavailability of hydrochlorothiazide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Capsules of valsartan, amlodipine and hydrochlorothiazide were prepared using the ingredients listed in Formulation 1 below.
[0063] Prescription 1
[0064] Valsartan 160g
[0065] Hydrochlorothiazide 12.5g
[0066] Amlodipine besylate 5g (Amlodipine meter)
[0067]
[0068] 1000 capsules
[0069] The valsartan, amlodipine and hydrochlorothiazide are respectively pulverized with a pulverizer (such as a jet mill) or a ball mill to a powder with a particle size of 100nm~60μm. Take prescription amount of valsartan, hydrochlorothiazide and amlodipine besylate powder, mix evenly, pack into capsules, and obtain the product.
Embodiment 2
[0071] Capsules of valsartan, amlodipine and hydrochlorothiazide were prepared using the ingredients listed in Formulation 2 below.
[0072] Prescription 2
[0073] Valsartan 160g
[0074] Hydrochlorothiazide 12.5 g
[0075] Amlodipine besylate 5g (Amlodipine meter)
[0076] Microcrystalline Cellulose 10g
[0077] Colloidal silicon dioxide 3g
[0078]
[0079] 1000 capsules
[0080]Grind valsartan, amlodipine and hydrochlorothiazide with a pulverizer (such as a jet mill) or a ball mill respectively to powders with a particle size of 100nm~60μm. Get the prescription amount of valsartan, hydrochlorothiazide and amlodipine besylate powder and mix evenly with auxiliary materials. Pack into capsules and get ready.
[0081]
Embodiment 3
[0083] Capsules of valsartan, amlodipine and hydrochlorothiazide were prepared using the ingredients listed in Formulation 3 below.
[0084] Prescription 3
[0085] Valsartan 160 g
[0086] Hydrochlorothiazide 12.5 g
[0087] Amlodipine besylate 5g (Amlodipine meter)
[0088] 10% starch slurry appropriate amount
[0089] Crospovidone 40g
[0090]
[0091] 1000 capsules
[0092] Grind valsartan, amlodipine and hydrochlorothiazide with a pulverizer (such as a jet mill) or a ball mill respectively to powders with a particle size of 100nm~60μm. Take prescription amount of valsartan, hydrochlorothiazide and amlodipine besylate powder and mix uniformly with auxiliary materials, and perform wet granulation to obtain dry granules. Pack into capsules to obtain capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com