Medicinal composition and preparation method thereof
A composition and drug technology, applied in the directions of drug combinations, active ingredients of heterocyclic compounds, cardiovascular system diseases, etc., can solve the problems of difficult industrial production, high energy consumption of dry granulation, high equipment requirements, and overcome mutual constraints. , The effect of smooth granulation process and low equipment requirements
Inactive Publication Date: 2012-07-04
BEIJING D VENTUREPHARM TECH DEV
View PDF2 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, dry granulation has the disadvantages of high energy consumption, small adaptability, high requirements for equipment or the need to renovate workshops, and certain dust pollution, making industrial production difficult.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
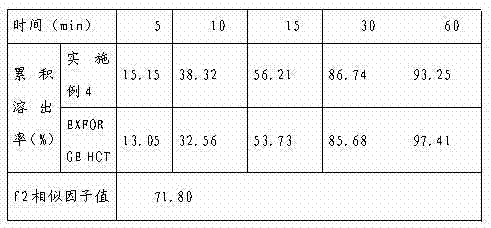
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
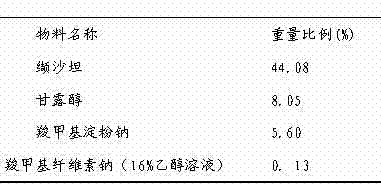
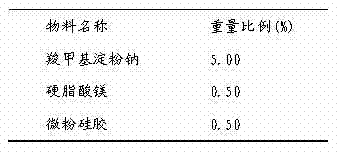
The invention discloses a medicinal composition. The medicinal composition consists of the following ingredients in percentage by weight: 1 to 5 percent of amlodipine and pharmaceutically acceptable salt thereof, 20 to 50 percent of valsartan, 1 to 10 percent of hydrochlorothiazide, 20 to 50 percent of filling agent, 8 to 24 percent of disintegrating agent, 0.01 to 0.06 percent of adhesive 1, 0.01 to 0.2 percent of adhesive 2, 0.3 to 2 percent of flow aid and 0.5 to 2.5 percent of lubricating agent. The medicinal composition has the advantages of simple preparation process, low equipment requirement, simplicity in operation and small dust pollution, guarantees the quality of medicines and contributes to industrialized production.
Description
technical field [0001] The invention relates to a pharmaceutical composition for treating hypertension and a preparation method thereof, in particular to a pharmaceutical composition containing amlodipine and a pharmaceutically acceptable salt thereof, valsartan and hydrochlorothiazide and a preparation method thereof. Background technique [0002] Hypertension is a common cardiovascular disease and can induce or aggravate other related diseases. At present, about 2 / 3 of hypertensive patients need to take two or more antihypertensive drugs to control blood pressure. Various clinical studies have shown that the use of drug combinations is one way to achieve desired therapeutic endpoints. The solid preparation of compound amlodipine valsartan hydrochlorothiazide is a safe and oral effective antihypertensive drug widely used in clinic. Amlodipine valsartan not only provides two powerful antihypertensive drugs, but also significantly reduces adverse reactions, and is convenie...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/549A61K31/4422A61K31/41A61K47/38A61K47/10A61P9/12
Inventor 黄雪吕青远
Owner BEIJING D VENTUREPHARM TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com