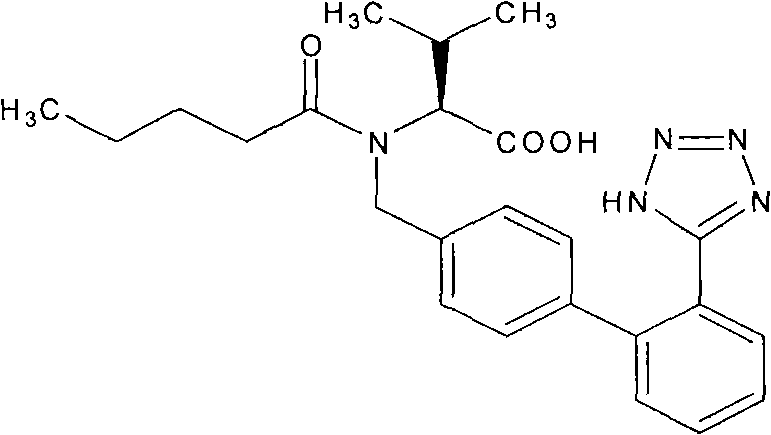
Valsartan and hydrochlorothiazide oral solid preparation with high medicament loading capacity
A technology for hydrochlorothiazide and solid preparation, applied in the field of pharmaceutical preparations and preparation thereof, can solve the problems of large dosage form and inconvenience for patients to take, and achieve the effects of good product stability, improved production efficiency and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
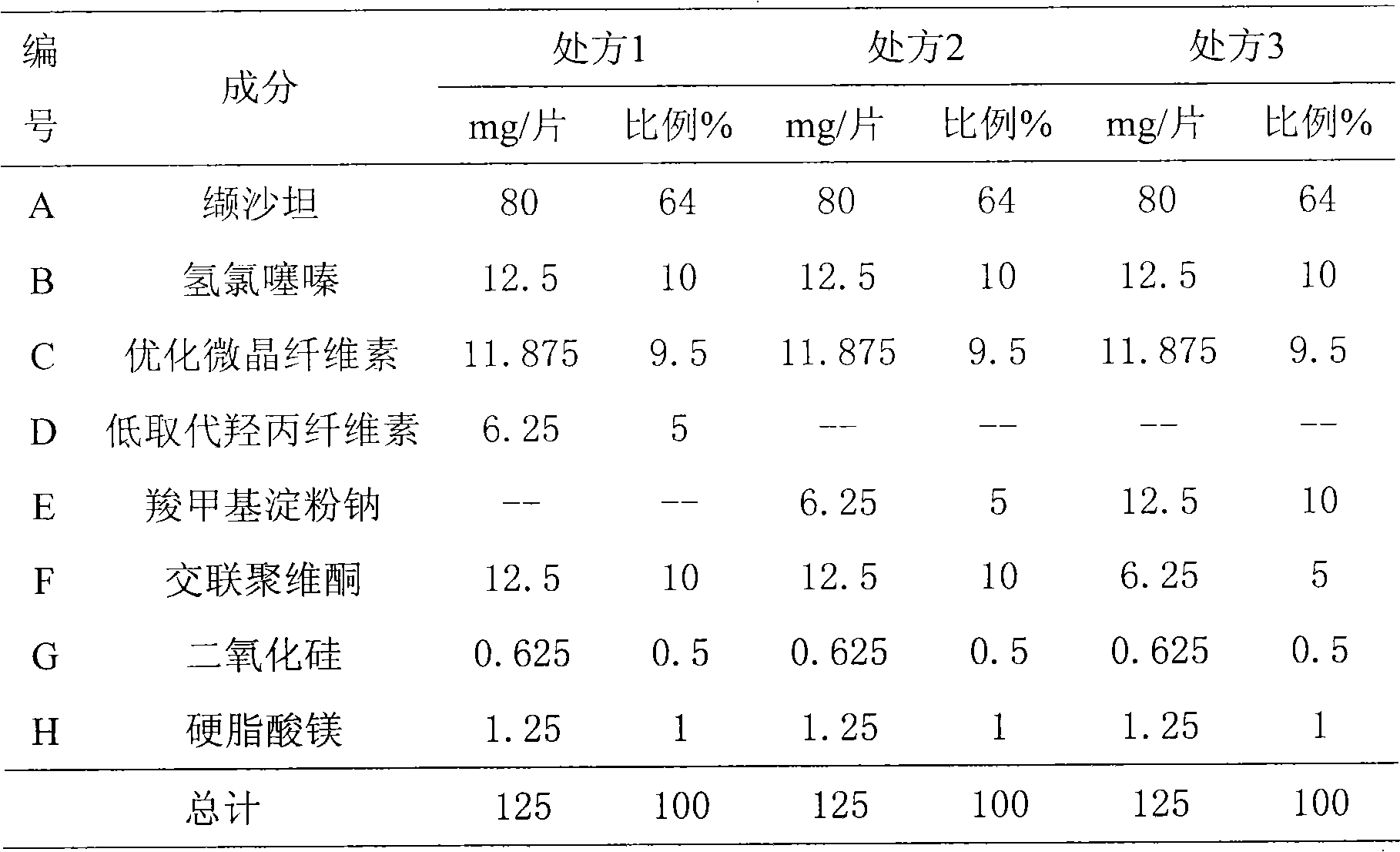
[0038] Preparation of Valsartan Hydrochlorothiazide Tablets, the material composition is shown in Table 1
[0039] Table 1 Material Composition Ratio
[0040]
[0041] Preparation of tablet cores: the prescribed amount of valsartan was granulated by roller compaction (Alexander WP120V), pressure: 70bar, roller speed: 3rpm. Mix the prepared granules with hydrochlorothiazide, optimized microcrystalline cellulose, low-substituted hydroxypropyl cellulose or sodium carboxymethyl starch, crospovidone, and silicon dioxide in a total mixing tank, then add magnesium stearate and mix Evenly, prepare 80 / 12.5mg valsartan-hydrochlorothiazide tablets according to the tablet weight, or double the tablet weight to prepare 160 / 25mg valsartan-hydrochlorothiazide tablets.
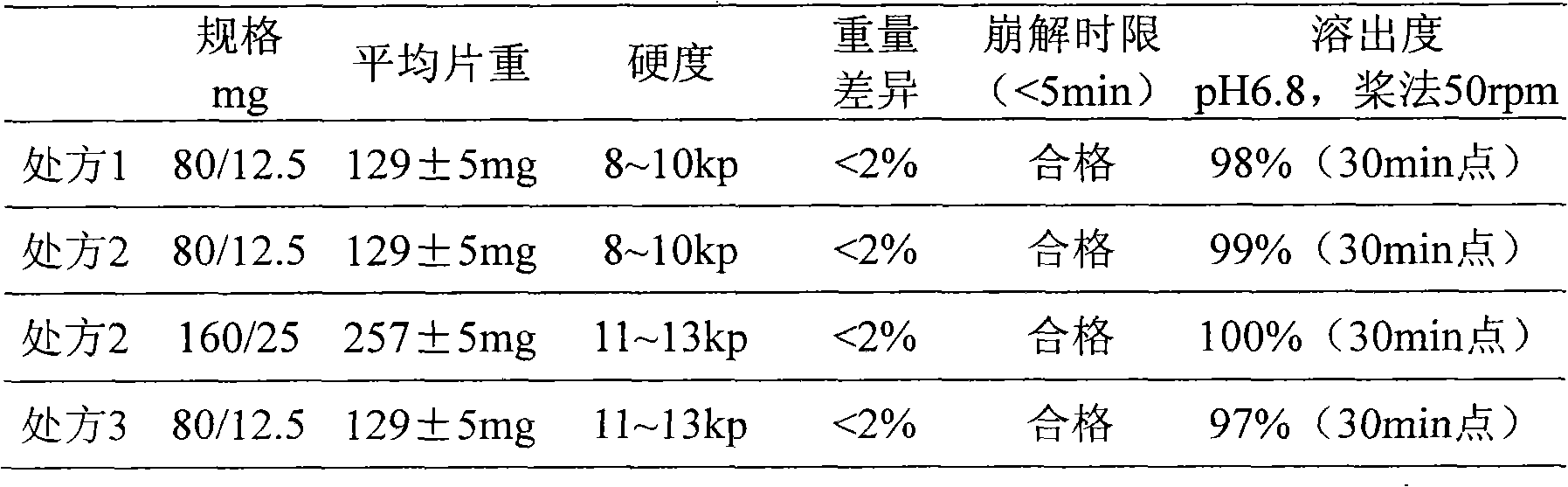
[0042] Preparation of coated tablets: Weigh Opadry prepared coating liquid, and coat the plain tablets. Until the sheet has gained about 3% in weight. Product quality is inspected, and the results are shown in Table 2 ...
Embodiment 2
[0046] Preparation of Valsartan Hydrochlorothiazide Tablets
[0047] The material composition is shown in Table 3
[0048] Table 3 Material Composition Ratio
[0049]
[0050] Preparation of tablet cores: the prescribed amount of valsartan was granulated by roller compaction (Alexander WP120V), pressure: 70bar, roller speed: 3rpm. Mix the prepared granules with hydrochlorothiazide, optimized microcrystalline cellulose, low-substituted hydroxypropyl cellulose or sodium carboxymethyl starch, crospovidone, and silicon dioxide in a total mixing tank, then add magnesium stearate and mix Evenly, according to the weight of the tablet, prepare 160 / 12.5 mg or 320 / 12.5 mg of valsartan hydrochlorothiazide tablets, or double the prescription 4-5 tablets and prepare 320 / 25 mg of valsartan and hydrochlorothiazide tablets.
[0051] Preparation of coated tablets: Weigh Opadry prepared coating liquid, and coat the plain tablets. Until the sheet has gained about 3% in weight. Product qua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com