Method and reagent box for predicting dihydrochlorothiazide antihypertensive efficacy
A technology of hydrochlorothiazide and a kit is applied in the field of medical molecular biology, which can solve the problems of reduced diuresis and antihypertensive effect, and achieve the effects of high representativeness, low cost and significant prediction effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Correlation study between SNP sites and hydrochlorothiazide antihypertensive efficacy
[0027] 1. Clinical trials:
[0028] Unrelated hypertensive patients aged 40-75 who had never received any hypertensive drug treatment or had not received any drug treatment in the last 8 weeks were enrolled from rural Xinyang, Henan. The diagnostic criteria for hypertension are systolic blood pressure ≥ 140mmHg and / or diastolic blood pressure ≥ 90mmHg at three different times within 2 months. The investigation process includes ①questionnaire basic information and medical history, traditional risk factors such as smoking and drinking, ②measurement of sitting blood pressure in both upper limbs, ③taking fasting venous blood samples, ④taking urine samples, ⑤measurement of height, weight, waist circumference, and hip circumference, ⑥Medical auscultation and physical examination, ⑦12-lead electrocardiogram, ⑧Echocardiography, ⑨Dispense medicine according to the random number, a...
Embodiment 2
[0093] Example 2: A kit for predicting the antihypertensive efficacy of hydrochlorothiazide
[0094]1. Composition: The composition and content are as follows (50 servings), stored at -20°C:
[0095] 50 μL 10×PCR buffer (TaKaRa),
[0096] 50μL 10mmol / L dNTP mixture (TaKaRa),
[0097] 12 μL (5U / uL) Taq DNA polymerase (TaKaRa),
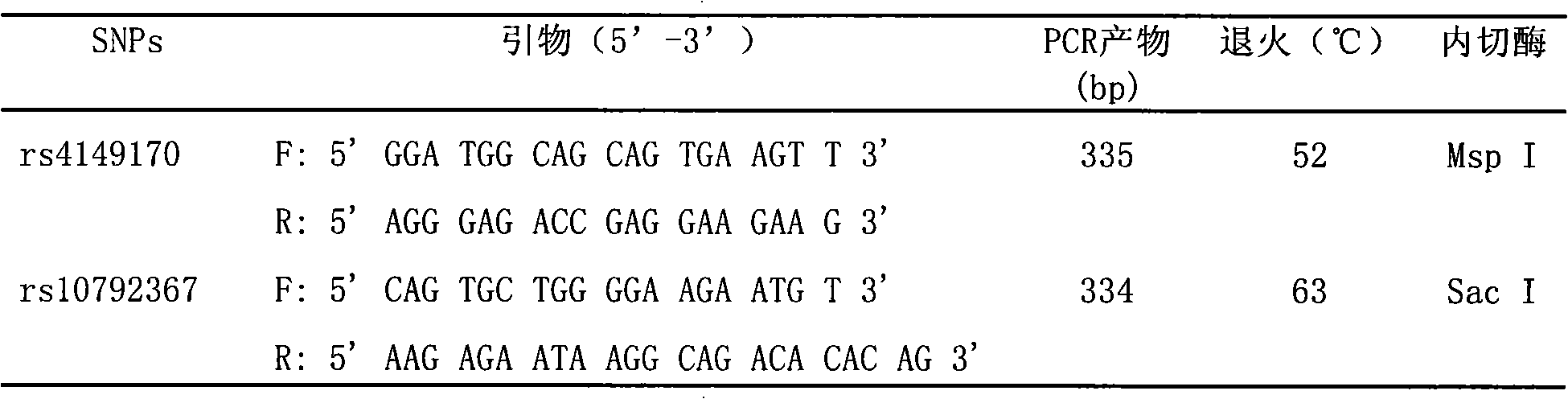
[0098] Each 20μL (10umol / L) of SEQ ID NO.1 and SEQ ID NO.2 primers (Beijing Aoke Biological Company)
[0099] 5ml deionized double distilled water (ddH2O),
[0100] 100 μL 10×Sac I restriction endonuclease reaction buffer (New England Biolabs, USA)
[0101] 35 μL (10U / μL) restriction endonuclease Sac I (New England Biolabs, USA)
[0102] 2. How to use
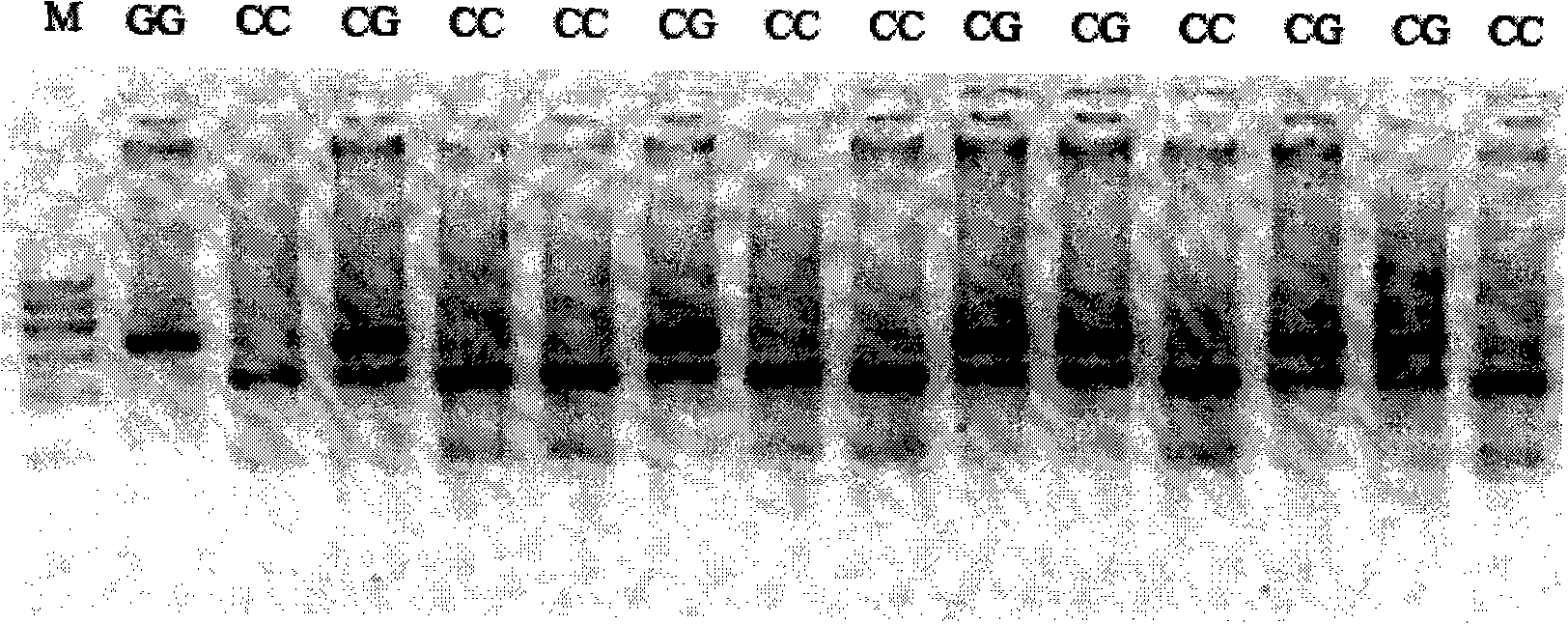
[0103] 1. Extract the subject's genomic DNA by conventional methods;
[0104] 2. PCR reaction:
[0105] Table 4: PCR reaction system
[0106]
[0107] Reaction conditions:
[0108] step 1=94.0℃ for 5 minutes
[0109]
[0110] step 5 = 72.0°C for 10 minutes
[0111] step 6=4.0℃
[0112]...
Embodiment 3
[0121] Example 3: Study on the predictive effect of the kit on the antihypertensive efficacy of hydrochlorothiazide
[0122] 1. In order to further verify the predictive effect of the kit on the antihypertensive effect of hydrochlorothiazide, 245 patients were selected in Shandong Rizhao according to the same inspection and standards as in Example 1, and 12.5 mg of hydrochlorothiazide was administered each time. Once a day, if the blood pressure is not up to standard after 2 weeks, the dose will be doubled, and the treatment will last for 8 weeks.
[0123] 2. Genotyping:
[0124] 1. The reagents and equipment used are exactly the same as in Example 1.
[0125] 2. Experimental steps:
[0126] (1) Genomic DNA was extracted from 4ml of peripheral blood using the Blood Genomic DNA Purification Kit (DP318-02), and all steps were performed according to the instructions.
[0127] (2) Genotype rs10792367 using the kit for predicting the antihypertensive efficacy of hydrochlorothiaz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com