Medicinal composition capsules of valsartan and hydrochlorothiazide and preparation method for capsules
A technology of hydrochlorothiazide and a composition, applied in the field of pharmaceutical preparations, can solve the problems of not disclosing the activity of soybean phosphatidylcholineylserine, affecting the combined use of valgetan and hydrochlorothiazide, and achieving liver protection, good therapeutic effect, and absorption capacity. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0017] Embodiment 2 Preparation of valsartan and hydrochlorothiazide pharmaceutical composition capsule
[0018] Weigh 8 g of hydrochlorothiazide, 60 g of phosphatidylcholine, 30 g of microcrystalline fiber, 8 g of polyacrylic acid resin NE30D, and 6 g of magnesium stearate. Mix hydrochlorothiazide, phosphatidylcholine, magnesium stearate and microcrystalline fiber evenly, and use 50% polyacrylic acid resin NE30D as a wetting agent / adhesive to prepare a soft material; put the soft material into the extruder Medium extrusion, the extrudate is placed in a spheronizer to make a pellet core, and dried to obtain a skeleton slow-release pellet core; the skeleton slow-release pellet core is placed in a fluidized bed, and the bottom spray method is used to spray polyacrylic acid resin NE30D coating to obtain slow-dissolving pellets.
[0019] Take by weighing valsartan 20g, PVPP 20g, microcrystalline cellulose 40g, sodium lauryl sulfate 3g, povidone K30 6g, magnesium stearate 3g, mi...
Embodiment 3
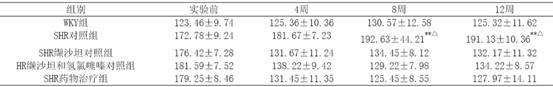
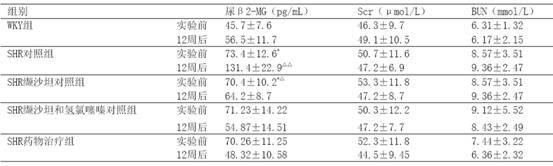
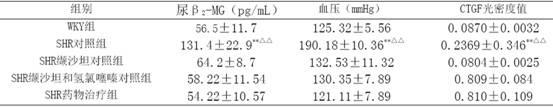
[0024] Example 3 Decompression and investigation of kidney protection
[0025] 40 12-week-old spontaneously hypertensive rats (SHR), 10 Wistar.Kyoto (WKY) rats of the same age, all male, were randomly divided into 3 groups, SHR control group (10 rats) SHR administration treatment group ( 10 rats) and the SHR valsartan control group (10 rats) and the SHR valsartan and hydrochlorothiazide control group (10 rats), first adaptively fed for 3 days, and then the valsartan control group received valsartan 30 mg / (kg· d) intragastric administration, SHR valsartan and hydrochlorothiazide were given a mixture of 22 mg valsartan and hydrochlorothiazide 8 mg / (kg d) intragastric administration, and the SHR drug treatment group adopted the drug group I described in Example 1 according to the valsartan Content of 15mg / (kg d), SHR control group and WKY group were given the same amount of distilled water orally every day, and the two kinds of rats were purchased from the Experimental Animal B...
Embodiment 4
[0047] Example 4 Comparison of Effects of Lowering Blood Pressure and Reversing Left Ventricular Hypertrophy
[0048] The 30 SHRs were randomly divided into 5 groups, 6 in each group, 1 group was the control group, and the remaining 4 groups were the medication groups. The medication groups were given valsartan 8mg / (kg·d) as the SHR valsartan control group, valsartan 8mg and hydrochlorothiazide 2mg mixture mg / (kg·d) as the SHR valsartan and hydrochlorothiazide control group and The drug group I described in Example 1 was used as the SHR drug treatment group according to the valsartan content of 8 mg / (kg·d). Rats were fed with normal pellet chow. Every day at 9 o'clock in the morning, the medicine was added with a certain amount of normal saline, and then intragastrically administered. Both the control group and the WKY group were intragastrically administered with the same amount of normal saline for 4 weeks.
[0049] The tail artery systolic pressure and body weight (BW)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com