Valsartan and hydrochlorothiazide composition, and its preparation method
A technology of hydrochlorothiazide and valsartan, which is applied in the field of compound tablets of valsartan and hydrochlorothiazide, which can solve the problems of high noise, large amount of dust produced by dry granulation, and insufficient popularity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
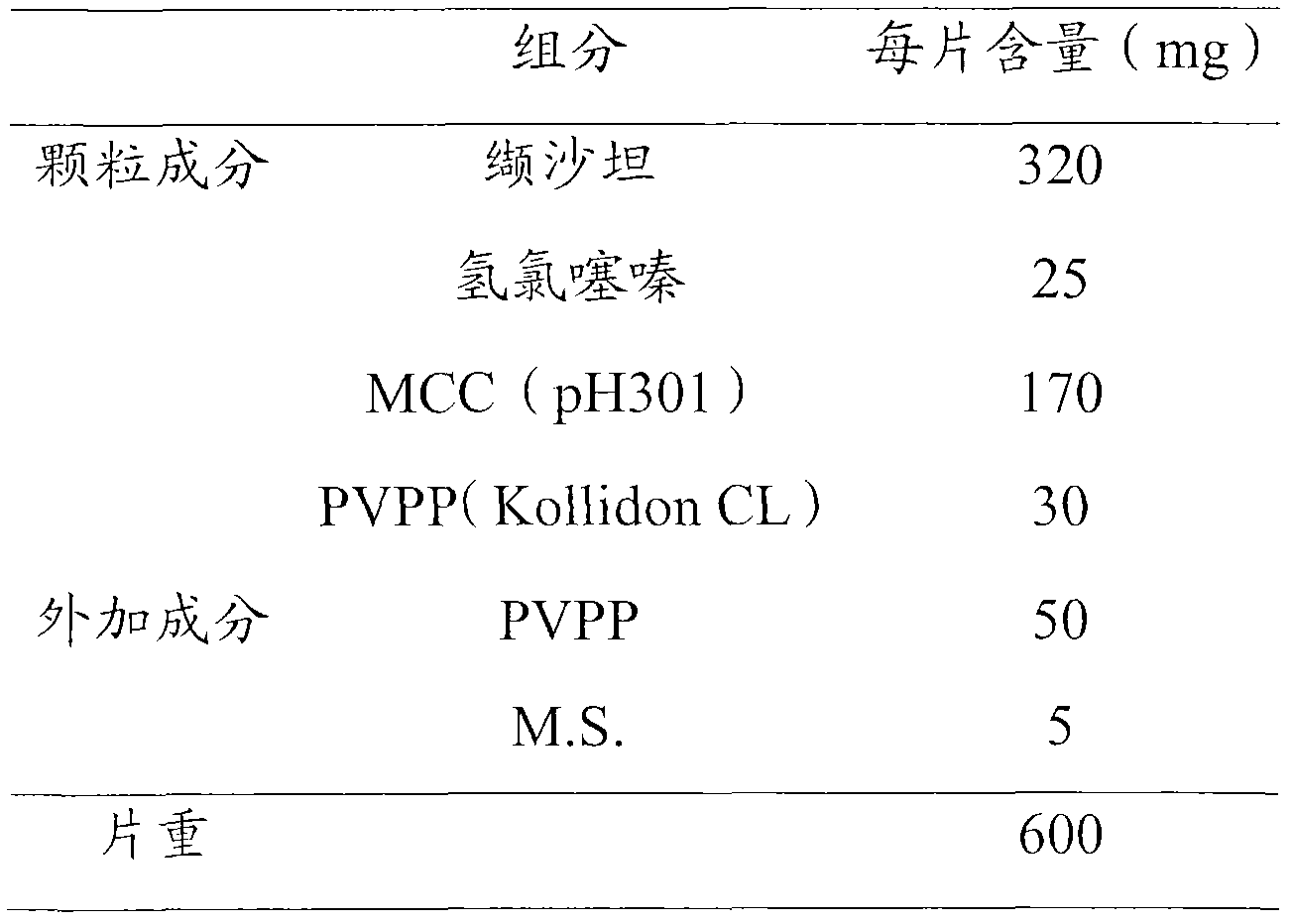
Embodiment 1
[0033]
[0034] Preparation Process:
[0035] i) Weigh the granule components and add them to the high-speed stirring granulator, start the agitation to make the mixture uniform; ii) add 10% ethanol-water solution to granulate; iii) use the fluidized bed to dry the obtained granules, and the drying temperature is controlled at 30-40 ℃; iv) passing the dried granules through a 20-mesh sieve for granulation; v) mixing the granules with additional auxiliary materials evenly; vi) pressing them into tablets.
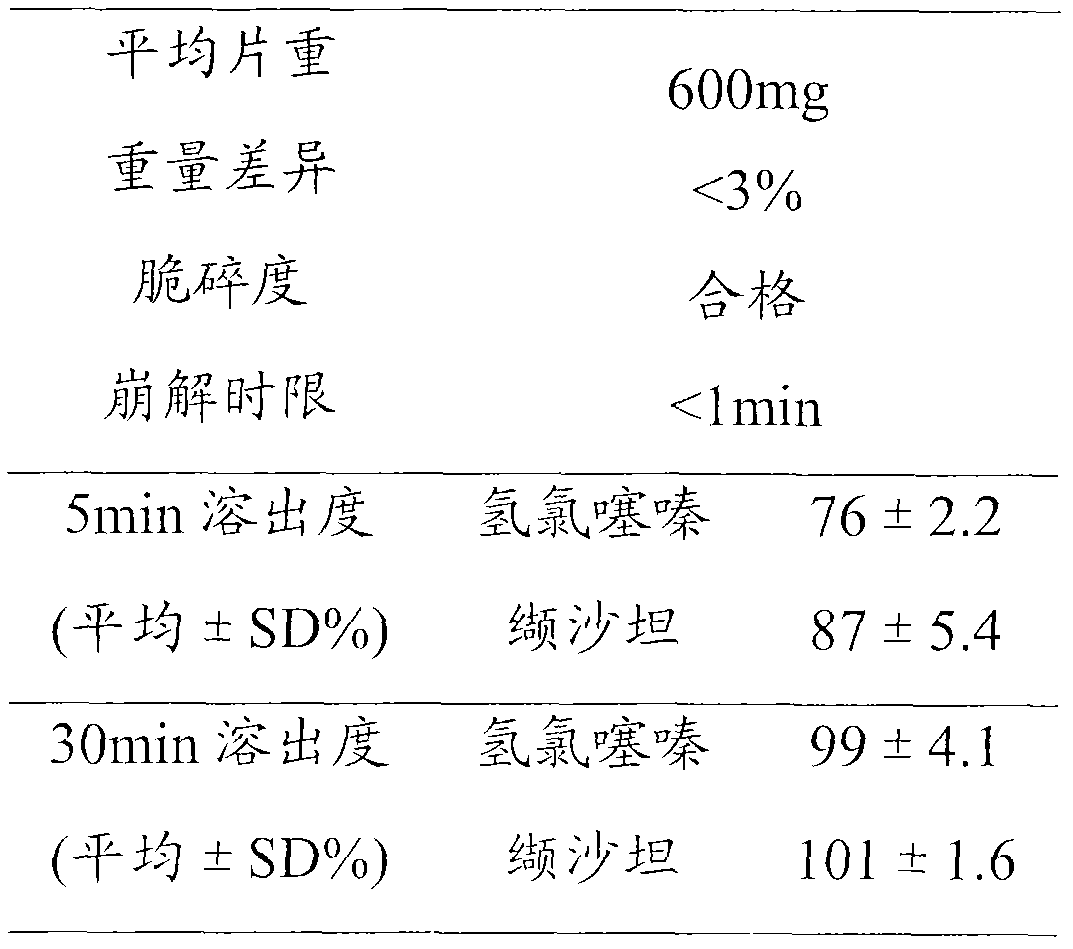
[0036]Carry out each detection according to the requirement of " Chinese Pharmacopoeia " 2010 editions, and wherein stripping condition is: adopt the device of dissolution assay method (Chinese Pharmacopoeia 2010 edition two appendices XC second method), take pH6.8 phosphate buffer saline 1000ml as Dissolution medium, the speed is 50r min -1 , operate according to the law, take 10ml of the solution at 5min and 30min respectively, filter with a 0.45μm microporous membrane,...
Embodiment 2
[0040]
[0041] Preparation process: i) weigh the granule components and add them to the high-speed stirring granulator, start the machine and stir to make the mixture even; ii) add 15% ethanol-water solution to granulate; iii) use the fluidized bed to dry the obtained granules, and the drying temperature is controlled at 30-40°C; iv) passing the dried granules through a 20-mesh sieve for granulation; v) mixing the granules with additional auxiliary materials evenly; vi) pressing them into tablets.
[0042] Test results:
[0043]
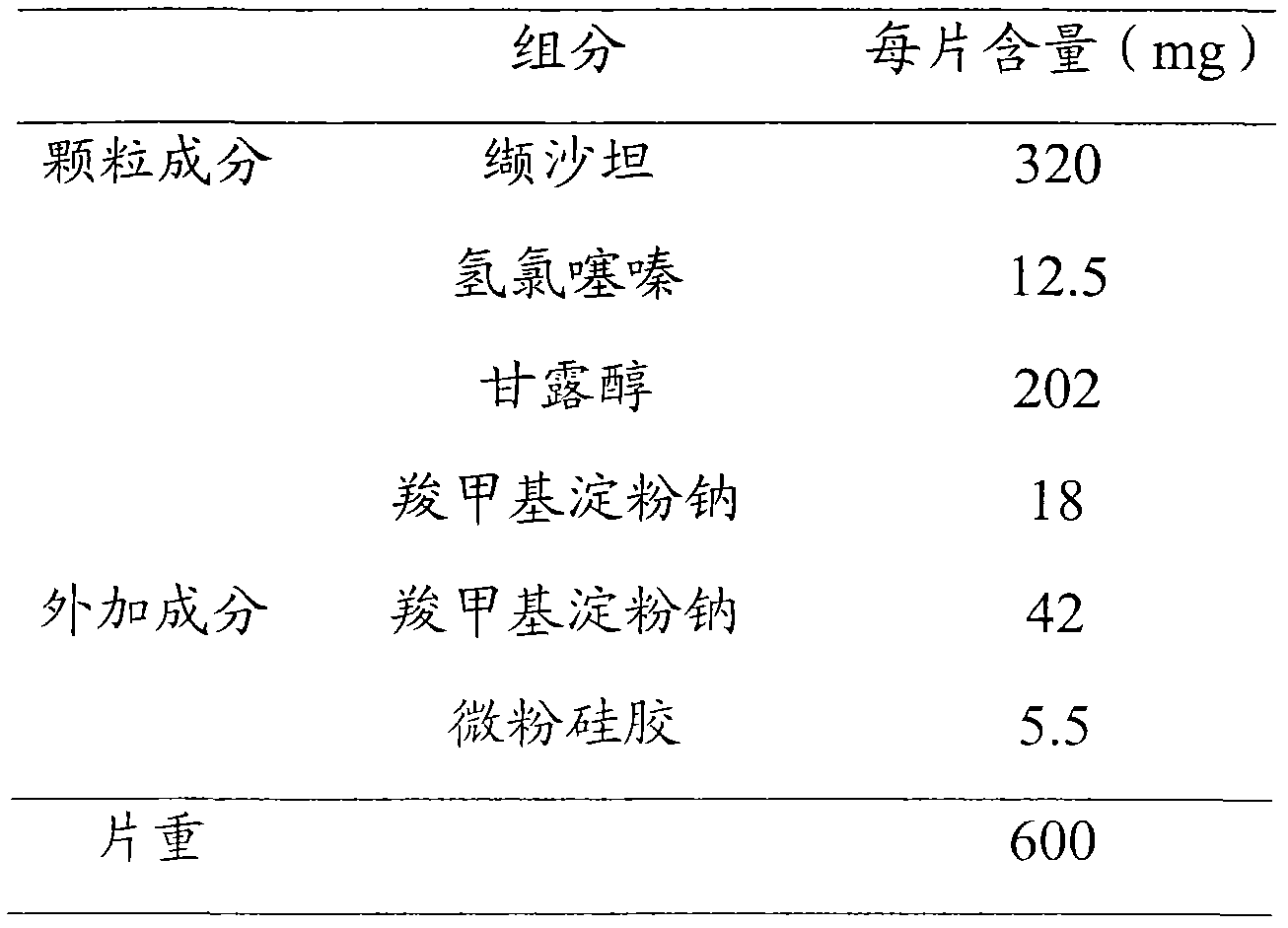
Embodiment 3
[0045]
[0046] Preparation process: i) Weigh the granule components and add them to the high-speed stirring granulator, start the machine and stir to mix evenly; ii) add 12% ethanol-water solution to granulate; iii) use the fluidized bed to dry the obtained granules, and the drying temperature is controlled at 30-40°C; iv) passing the dried granules through a 20-mesh sieve for granulation; v) mixing the granules with additional auxiliary materials evenly; vi) pressing them into tablets.
[0047] Test results:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com