Compound amlodipine and valsartan solid preparation and preparation method thereof
A technology for compound amlodipine-valsartan solid preparation is applied in the field of compound amlodipine-valsartan solid preparation and preparation thereof, and can solve the problems of affecting the dissolution of amlodipine, the disparity of amlodipine doses, and the high energy consumption of dry granulation , to achieve the effect of good liquidity, good industrial production value, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
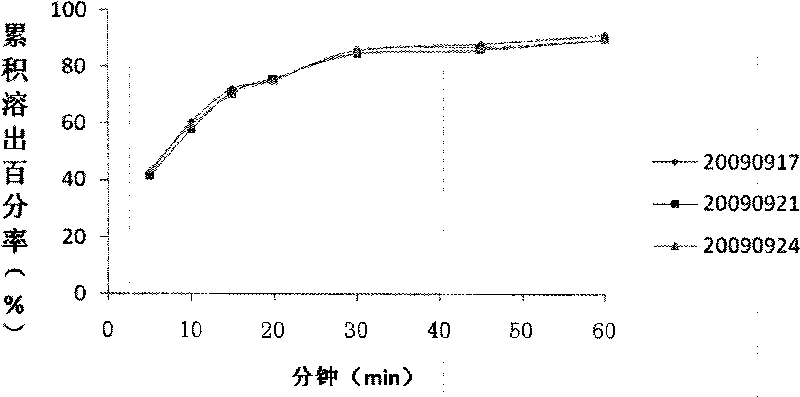
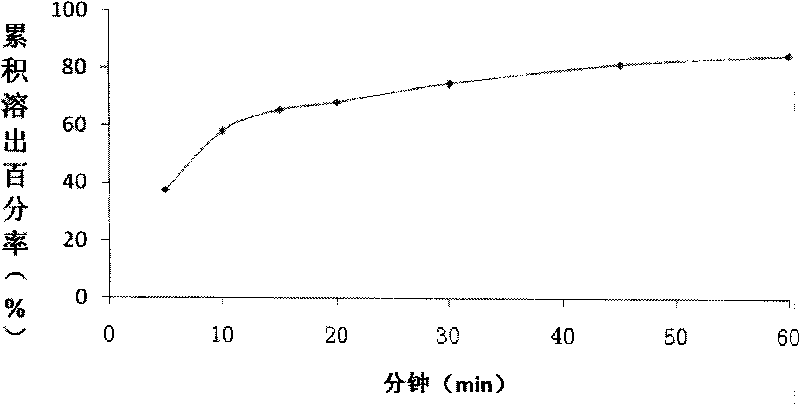
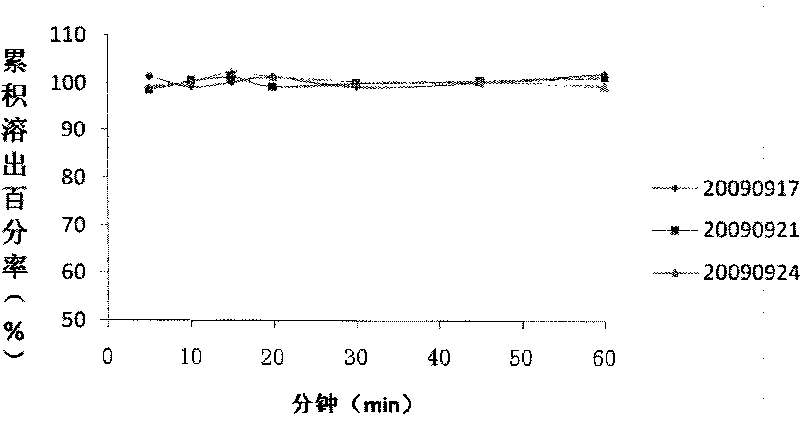
Image
Examples
Embodiment 1
[0062] Preparation of Compound Amlodipine-Valsartan Tablets
[0063] The prescription is (per 1000 tablets):
[0064] A: Amlodipine besylate 6.93g *
[0065] B: micronized silica gel 5g
[0066] C: Croscarmellose Sodium 8g
[0067] D: Valsartan 160g
[0068] E: Microcrystalline Cellulose 110g
[0069] F: Lactose 50g
[0070] G: Magnesium stearate 2g
[0071] H: Croscarmellose Sodium 4g
[0072] I: Polyvinylpyrrolidone (K30) 37g
[0073] J: distilled water 900g
[0074] *: Corresponds to 5 g of amlodipine.
[0075] Grind materials A~C, pass through a 100-mesh sieve, and mix uniformly to obtain mixture I; crush materials D~F, pass through a 100-mesh sieve, and mix evenly to obtain mixture II; add mixture I to In mixture II, mix evenly to obtain mixture III; completely dissolve I: polyvinylpyrrolidone (K30) in J: distilled water to obtain a binder; use this binder to granulate mixture II, and control the drying temperature at 45 °C, the water content of the granules i...
Embodiment 2
[0079] Preparation of compound amlodipine valsartan besylate capsules
[0080] The prescription is (per 1000 capsules):
[0081] A: Amlodipine besylate 6.93g *
[0082] B: micronized silica gel 5g
[0083] C: Croscarmellose Sodium 8g
[0084] D: Valsartan 160g
[0085] E: Microcrystalline Cellulose 110g
[0086] F: Lactose 50g
[0087] G: Magnesium stearate 4g
[0088] H: medicinal talcum powder 2g
[0089] I: Polyvinylpyrrolidone (K30) 37g
[0090] J: distilled water 900g
[0091] *: Corresponds to 5 g of amlodipine.
[0092] Grind materials A~C, pass through a 100-mesh sieve, and mix uniformly to obtain mixture I; crush materials D~F, pass through a 100-mesh sieve, and mix evenly to obtain mixture II; add mixture I to the mixture in equal increments In II, mix evenly to obtain mixture III; completely dissolve I: polyvinylpyrrolidone (K30) in H: distilled water to obtain a binder; use this binder to granulate mixture II, and control the drying temperature at 45°C ...
Embodiment 3
[0095] Preparation of Compound Amlodipine-Valsartan Tablets
[0096] The prescription is (per 1000 tablets):
[0097] A: Amlodipine besylate 3.47g *
[0098] B: micronized silica gel 4g
[0099] C: Crospovidone 6g
[0100] D: Valsartan 80g
[0101] E: Microcrystalline cellulose 80g
[0102] F: Lactose 30g
[0103] G: Magnesium stearate 2g
[0104] H: Low-substituted hydroxypropyl cellulose 2g
[0105] I: Hypromellose (E4M) 21g
[0106] J: Distilled water 700g
[0107] *: Corresponds to 2.5 g of amlodipine.
[0108]Grind materials A~C, pass through a 100-mesh sieve, and mix uniformly to obtain mixture I; crush materials D~F, pass through a 100-mesh sieve, and mix evenly to obtain mixture II; add mixture I to In mixture II, mix evenly to obtain mixture III; completely dissolve I: polyvinylpyrrolidone (K30) in J: distilled water to obtain a binder; use this binder to granulate mixture II, and control the drying temperature at 45 °C, the water content of the granules i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com