Low-concentration tropane drug eye drops and preparation method
A low-concentration, eye-drop technology, applied in the field of pharmaceuticals, can solve problems such as follicular conjunctivitis, eyelid skin eczema, and eye margin congestion and erosion, local allergic reactions, poor patient compliance, and affecting treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
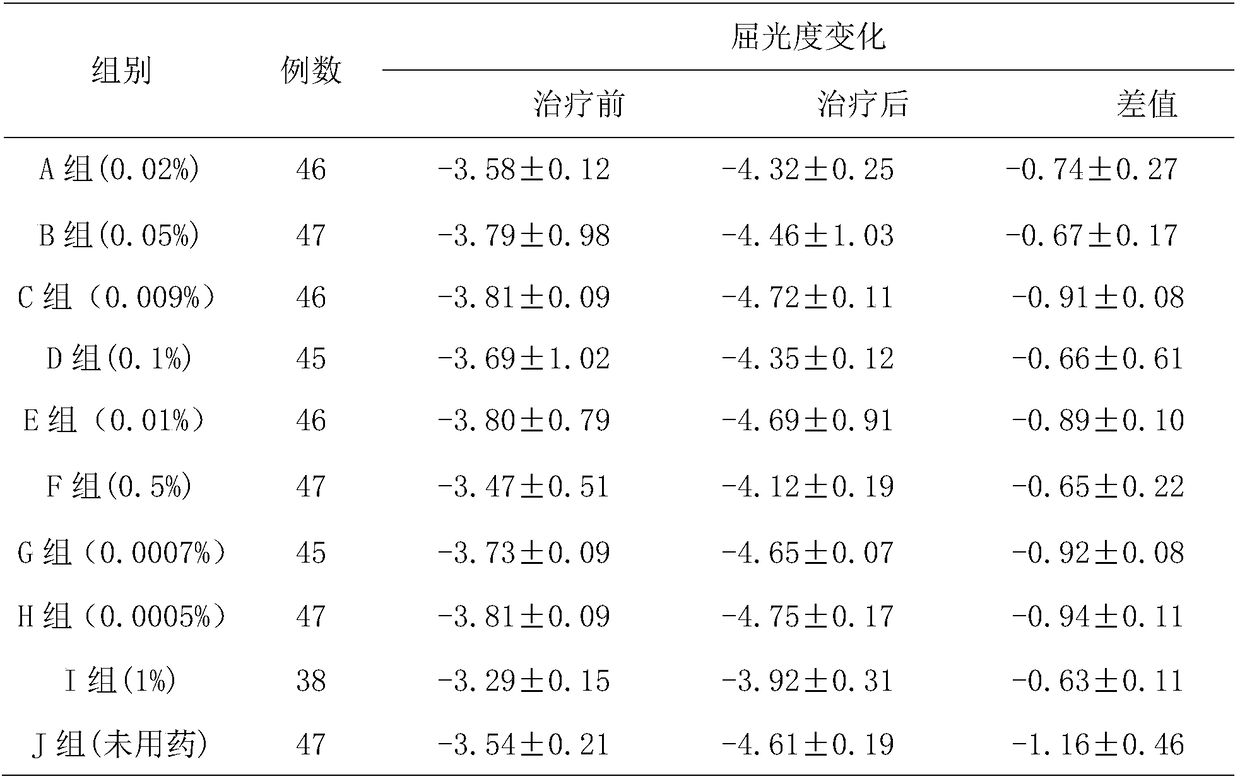
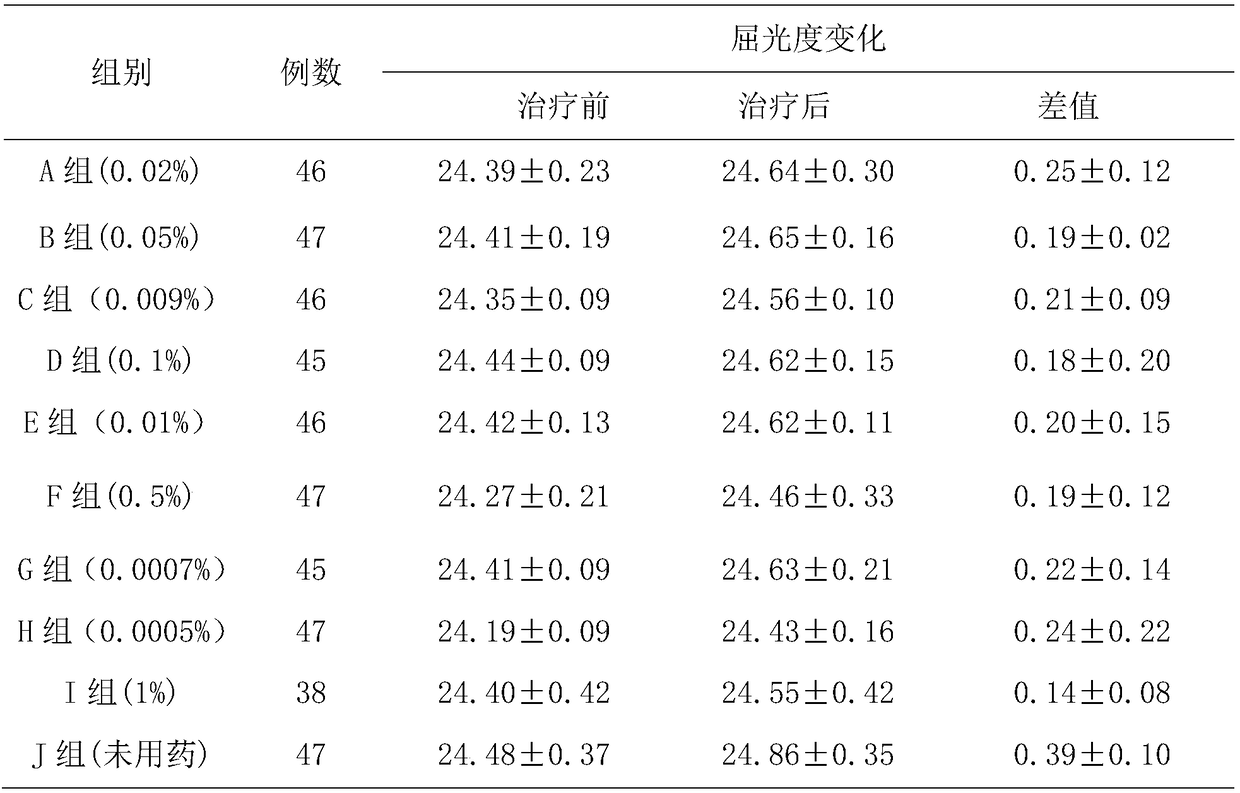
[0031] The present embodiment provides a kind of low-concentration tropane drug eye drops, which comprises the following components: atropine sulfate 0.02% (w / v), sodium hyaluronate 0.35% (w / v), sodium chloride 0.75% (w / v), ethylparaben 0.01% (w / v), adjust the pH to 4.6 with phosphate buffer, and the balance is water for injection.
[0032] The preparation method of this low-concentration tropane drug eye drop comprises the steps:
[0033] S1. Weigh ethylparaben according to the proportion and dissolve it in water for injection at 75°C to obtain solution 1;
[0034] S2. Take sodium chloride according to the proportion and dissolve it in solution 1, and cool to obtain solution 2;
[0035] S3, taking sodium hyaluronate according to the proportion and fully dissolving it in solution 2 to obtain solution 3;
[0036] S4, take sulfuric acid by proportioning and take tropine and dissolve in solution 3 to obtain solution 4;
[0037] S5. Filter the solution 4, add water for injectio...
Embodiment 2
[0039] The present embodiment provides a low-concentration tropane drug eye drop, which comprises the following components: atropine sulfate 0.05% (w / v), sodium hyaluronate 0.01% (w / v), β-cyclodextrin 0.3% (w / v), sodium chloride 0.90% (w / v), ethylparaben 0.05% (w / v), adjust the pH to 6.2 with phosphate buffer, and the balance is water for injection.
[0040] The preparation method of this low-concentration tropane drug eye drop comprises the steps:
[0041] S1. Weigh β-cyclodextrin according to the proportion and dissolve it in water for injection, then weigh atropine sulfate and add it, place it in an ultrasonic vibration cleaning machine, and mix ultrasonically for 30 minutes, so that the atropine sulfate is completely clathrated by β-cyclodextrin, Obtain solution 1;
[0042] S2. Weigh the ethylparaben according to the proportion and dissolve it in water for injection at 40°C to obtain solution 2;
[0043] S3. Take sodium chloride according to the proportion and dissolve i...
Embodiment 3
[0048] The present embodiment provides a low-concentration tropane drug eye drop, which comprises the following components: atropine sulfate 0.0009% (w / v), sodium hyaluronate 0.05% (w / v), β-cyclodextrin 2% (w / v), sodium chloride 0.86% (w / v), ethylparaben 0.05% (w / v), adjust the pH to 5.8 with phosphate buffer, and the balance is water for injection.
[0049] The preparation method of this low-concentration tropane drug eye drop comprises the steps:
[0050] S1. Weigh β-cyclodextrin according to the proportion and dissolve it in water for injection, then weigh atropine sulfate and add it, place it in an ultrasonic vibration cleaning machine, and mix ultrasonically for 30 minutes, so that the atropine sulfate is completely clathrated by β-cyclodextrin, Obtain solution 1;
[0051] S2. Weigh the ethylparaben according to the proportion and dissolve it in water for injection at 45°C to obtain solution 2;
[0052]S3. Take sodium chloride according to the proportion and dissolve it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com