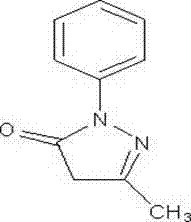
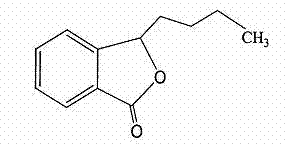
Butylphthalide- and edaravone-containing compound injection and preparation method thereof
A technology of injection and butylphthalide, which is applied in the field of medicine, can solve problems such as unadjusted dosage, elevated ALT or AST, and increased liver adverse reactions, so as to reduce the metabolic burden of the liver, reduce the dosage of the drug, and improve the quality of the liver. The effect of reducing the recurrence rate of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 50g of racemic butylphthalide, 50g of edaravone, 300g of hydroxypropyl-β-cyclodextrin, 900g of sodium chloride, 20g of sodium bisulfite, 200ml of absolute ethanol.
[0036] Preparation:
[0037] Step a, inclusion:
[0038] ① Prepare the absolute ethanol solution of racemic butylphthalide for subsequent use;
[0039] ②Take 2L of water for injection, heat it to 45°C, add hydroxypropyl-β-cyclodextrin, stir to dissolve completely, then add the solution obtained in step ① at 0.5ml / min, and stir until it becomes clear and transparent without oil drops exist, then add water for injection to 80L, and the resulting solution is ready for use;
[0040] Step b, preparation of compound solution: add disodium hydrogen phosphate and sodium dihydrogen phosphate (ratio 1:1) to the solution obtained in step a, add appropriate amount of phosphoric acid to control pH4.0, then add 20g of sodium bisulfite, chloride Sodium 900g, stir to make it dissolve completely, finally add edaravone, s...
Embodiment 2
[0044] D-butylphthalide 25g, Edaravone 25g, hydroxypropyl-β-cyclodextrin 800g, sodium chloride 700g, L-cysteine 10g, absolute ethanol 200ml.
[0045] Preparation:
[0046] Step a, inclusion:
[0047] ①Prepare the dehydrated ethanol solution of D-butylphthalide for subsequent use;
[0048] ②Take 4L of water for injection, heat to 50°C, add hydroxypropyl-β-cyclodextrin, stir to dissolve completely, then add the solution obtained in step ① at 5ml / min, and stir until it becomes clear and transparent without oil droplets. Then add water for injection to 60L, and the resulting solution is ready for use;
[0049]Step b. Prepare compound solution: add disodium hydrogen phosphate and sodium dihydrogen phosphate (ratio 5:1) to the solution obtained in step a, add appropriate amount of phosphoric acid to control pH4.5, then add 10g of L-cysteine, Sodium chloride 700g, stir to make it dissolve completely, finally add Edaravone, stir to make it dissolve completely in the solution, the...
Embodiment 3
[0053] 50g of L-butylphthalide, 25g of Edaravone, 800g of hydroxypropyl-β-cyclodextrin, 855g of sodium chloride, 50g of acetylcysteine, 500ml of absolute ethanol.
[0054] Preparation:
[0055] Step a, inclusion:
[0056] ①Prepare the dehydrated ethanol solution of L-butylphthalide for subsequent use;
[0057] ②Take 4L of water for injection, heat to 50°C, add hydroxypropyl-β-cyclodextrin, stir until it dissolves completely, then add the solution obtained in step ① at 10ml / min, stir until it becomes clear and transparent, without oil droplets, Then add water for injection to 60L, and the resulting solution is ready for use;
[0058] Step b. Prepare compound solution: add disodium hydrogen phosphate and sodium dihydrogen phosphate (ratio 10:1) to the solution obtained in step a, add appropriate amount of phosphoric acid to control pH3.0, then add acetylcysteine 50g, chlorine Sodium chloride 855g, stir to make it dissolve completely, finally add Edaravone, stir to make it d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com