Medicinal composition containing caffeic acid ester and scutellarin, preparation method and application thereof
A technology of breviscapine and caffeic acid ester, applied in the field of medicinal preparations, can solve the problems of unstable preparations, excessive related substances, imperfect processes, etc., and achieve the effect of accurately removing impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] A medicinal composition containing caffeic acid ester and scutellarin, which is in the form of aqueous injection.
[0148] The weight ratio of caffeic acid ester and scutellarin contained in the composition is 1.2:1~19:1 (1.2:1~5.5:1);
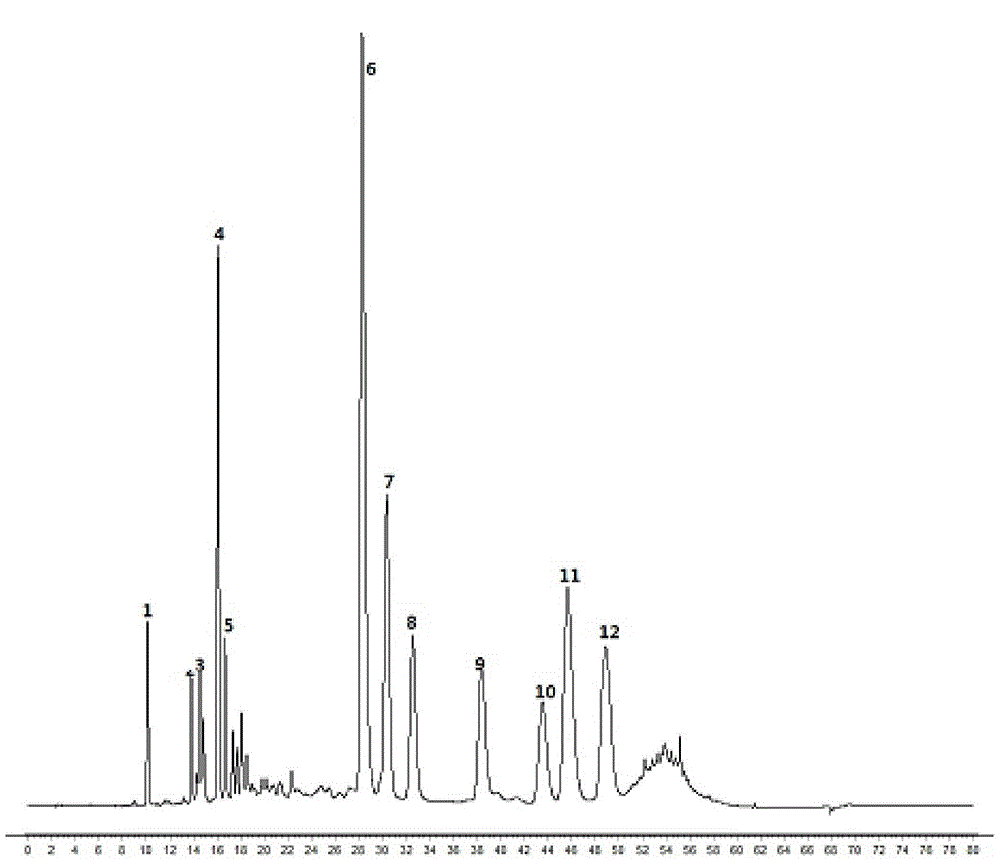
[0149] Taking 10ml of injection as the unit of measurement, the dry matter content is 115-145 mg, the dry matter content of total caffeic acid esters and scutellarin is 8.2-20.0 mg (average value 12.5 mg); dicaffeoylquinic acid 7.2- 14.0mg (average value 10.6mg); wherein, the content of 3,4-O-dicaffeoylquinic acid in the dicaffeoylquinic acid is 1.4-2.3mg (average value 1.873mg), 3,5-O - dicaffeoylquinic acid 0.8-1.4mg (average value 1.063mg), 4,5-O-dicaffeoylquinic acid 1.5-2.6mg (average value 2.079mg), phenanthridine 1-2.6mg (average value Value 1.828mg), scutellarin 2.2-5.1mg (average value 3.619mg).
[0150] The caffeic acid ester and scutellarin are prepared by the following steps:
[0151] Take 800 g of Erigeron lanceolata, ad...
Embodiment 2
[0154] A medicinal composition containing caffeic acid ester and scutellarin, which is an injection.
[0155] The weight ratio of caffeic acid ester and scutellarin contained in the composition is 5.5:1;
[0156] Taking 10ml of injection as the unit of measurement, the dry matter content is 115-145 mg, the dry matter content of total caffeic acid esters and scutellarin is 8.2-20.0 mg (average value 12.5 mg); dicaffeoylquinic acid 7.2- 14.0mg (average value 10.6mg); Wherein, the content of 3,4-O-dicaffeoylquinic acid in the dicaffeoylquinic acid is 1.4-2.3mg (average value 1.873mg), 3,5-O Two-caffeoylquinic acid 0.8-1.4mg (average value 1.063mg), 4,5-O-dicaffeoylquinic acid 1.5-2.6mg (average value 2.079mg), 1-2.6mg (average value) Value 1.828mg), scutellarin 2.2-5.1mg (average value 3.619mg).
[0157] The caffeic acid ester and scutellarin are prepared by the following steps:
[0158] Take 800 g of Erigeron lanceolata, add water to decoct at least twice, combine the decocti...
Embodiment 3
[0160] A medicinal composition containing caffeic acid ester and scutellarin, which is an injection.
[0161] The weight ratio of caffeic acid ester and scutellarin contained in the composition is 1.2:1;
[0162] Taking 10ml of injection as the unit of measurement, the dry matter content is 115-145 mg, the dry matter content of total caffeic acid esters and scutellarin is 8.2-20.0 mg (average value 12.5 mg); dicaffeoylquinic acid 7.2- 14.0mg (average value 10.6mg); wherein, the content of 3,4-O-dicaffeoylquinic acid in the dicaffeoylquinic acid is 1.4-2.3mg (average value 1.873mg), 3,5-O - dicaffeoylquinic acid 0.8-1.4mg (average value 1.063mg), 4,5-O-dicaffeoylquinic acid 1.5-2.6mg (average value 2.079mg), phenanthridine 1-2.6mg (average value Value 1.828mg), scutellarin 2.2-5.1mg (average value 3.619mg).
[0163] The caffeic acid ester and scutellarin are prepared by the following steps:
[0164] Take 800 g of Erigeron lanceolata, add water to decoct at least twice, combi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com