Clonidine hydrochloride multivesicular liposome and preparation method thereof
A technology of clonidine hydrochloride and multivesicular liposomes, which is applied in liposome delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of unsuitable multivesicular liposome prescriptions and preparation processes, etc. Achieve the effects of reducing adverse reactions, reducing the number of medications, and good sustained-release effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step 1: Accurately weigh 100 mg of lecithin, 77 mg of cholesterol, and 3.2 mg of glyceryl trioleate, dissolve and dilute to 5 ml with chloroform-ether (1:1; v / v), as the lipid phase;
[0046] Step 2: Accurately weigh 25 mg of clonidine hydrochloride and 400 mg of sucrose, ultrasonically dissolve with an appropriate amount of 80 mmol / L citric acid solution, and use the citric acid solution to dilute to 5 ml as the inner water phase;
[0047] Step 3: Slowly add the above-mentioned internal water phase to the upper layer of the lipid phase, and use a high-speed shear homogenizer to act on it at a speed of 13,000 rpm for 8 minutes to obtain a water-in-oil emulsion;
[0048] Step 4: Add the emulsion prepared in step 3 to 25ml of the external aqueous phase containing 3.2mg / ml glucose and 40mmol / L lysine, and use a high-speed shear homogenizer to act on it at a speed of 10,000rpm for 8 seconds Clock, forming water-in-oil-in-water type double emulsion;
[0049] Step 5: inject ...
Embodiment 2
[0052] Step 1: Precisely weigh 100 mg of lecithin, 26 mg of cholesterol, and 3.2 mg of glycerol trioleate, dissolve it in chloroform and set the volume to 5 ml, and use it as the lipid phase;
[0053] Step 2: Accurately weigh 75 mg of clonidine hydrochloride and 50 mg of sucrose, ultrasonically dissolve with an appropriate amount of 30 mmol / L citric acid solution, and use the citric acid solution to dilute to 5 ml as the inner water phase;
[0054] Step 3: with embodiment 1, make water-in-oil type emulsion;
[0055] Step 4: Add the emulsion prepared in step 3 to 25ml of the external aqueous phase containing 3.2mg / ml glucose and 20mmol / L lysine, and use a high-speed shear homogenizer to act on it at a speed of 10000rpm for 8 seconds Clock, forming water-in-oil-in-water type double emulsion;
[0056] Step 5: Same as Example 1, multivesicular liposome suspension.
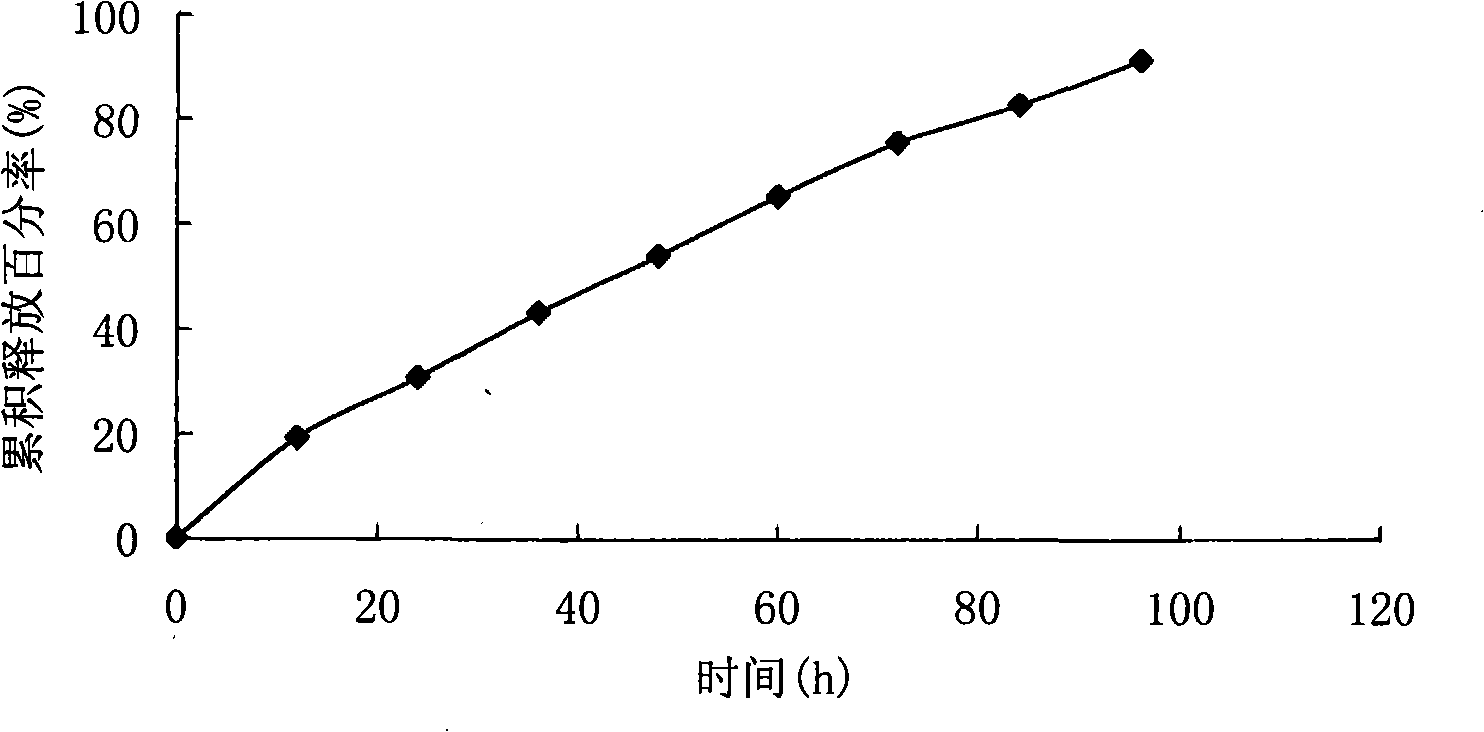
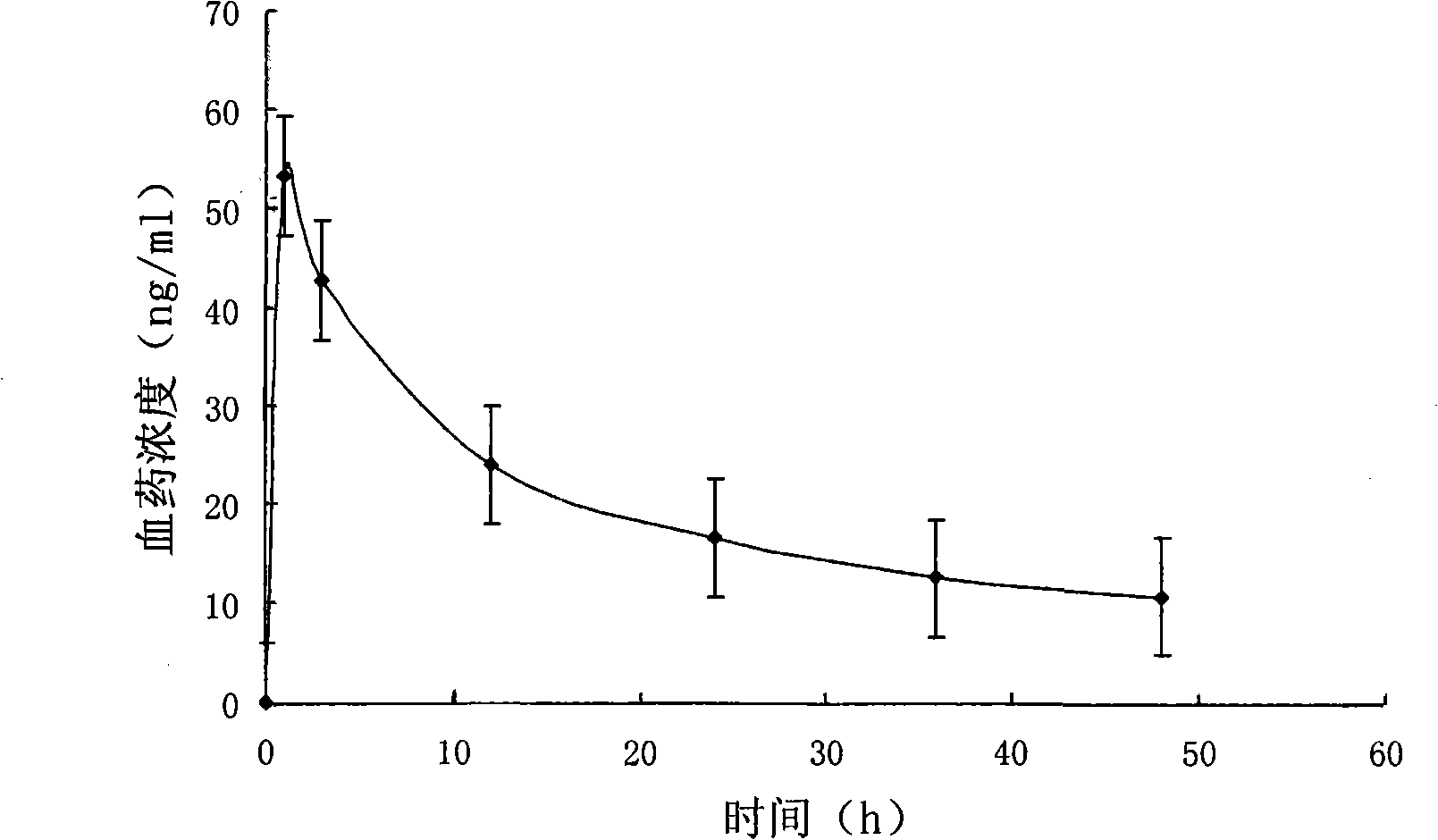
[0057] The particle size of clonidine hydrochloride multivesicular liposomes was measured to be 8-45 μm; the encap...
Embodiment 3
[0059] Step 1: Accurately weigh 200 mg of lecithin, 77 mg of cholesterol, 30 mg of phosphatidylserine and 6.4 mg of glyceryl trioleate, dissolve and dilute to 5 ml with chloroform-ether (1:1, v / v) as the lipid phase;
[0060] Step 2: Accurately weigh 75mg of clonidine hydrochloride and 300mg of sucrose, ultrasonically dissolve with an appropriate amount of 100mmol / L hydrochloric acid solution, and use the citric acid solution to dilute to 10.0ml as the inner water phase;
[0061] Step 3: with embodiment 1, make water-in-oil type emulsion;
[0062] Step 4: Add the emulsion prepared in step 3 to 25ml of the external aqueous phase containing 3.2mg / ml glucose and 80mmol / L lysine, and use a high-speed shear homogenizer to act on it at a speed of 10000rpm for 8 seconds Clock, forming water-in-oil-in-water type double emulsion;
[0063] Step 5: Same as Example 1, multivesicular liposome suspension.
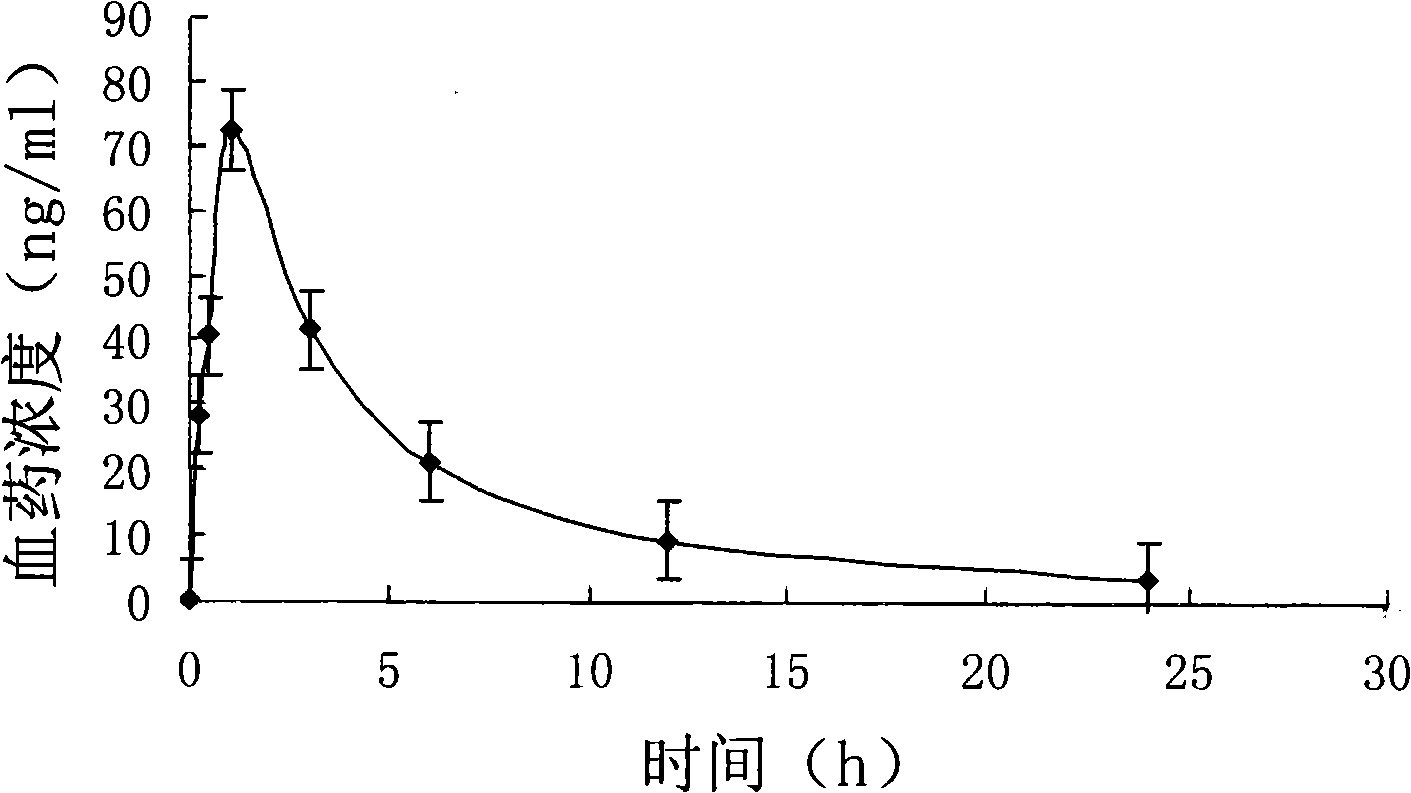
[0064] The particle size of clonidine hydrochloride multivesicular liposomes was m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com