Method for preparing clonidine hydrochloride
A technology of clonidine hydrochloride and formic acid, applied in the field of medicine and chemical industry, can solve the problems of low purity and low yield of clonidine hydrochloride, and achieve the effects of reducing cost, energy consumption and pollution, improving purity and yield, and having strong controllability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
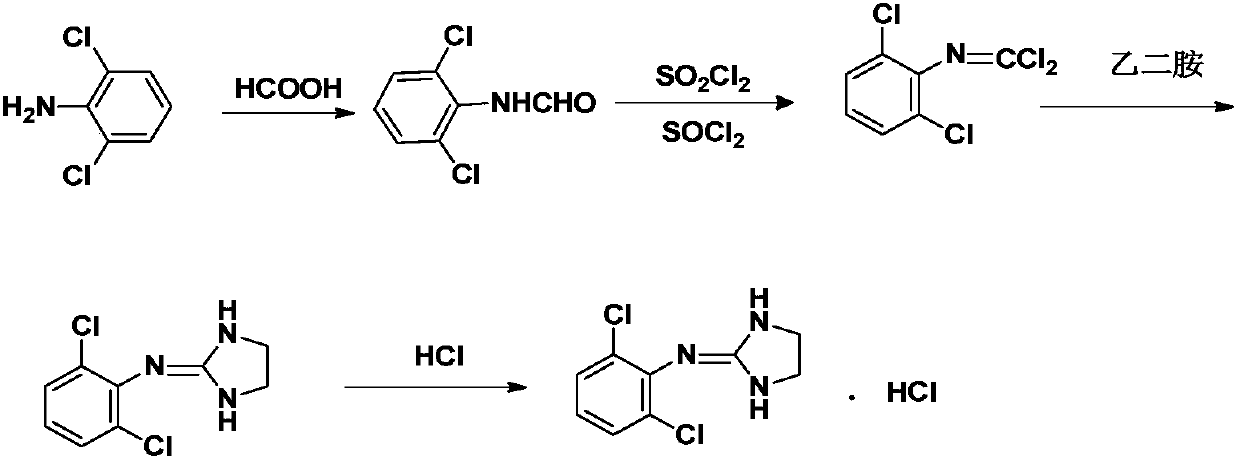
[0045] A. Synthesis of Intermediate 1
[0046] Add 188ml of formic acid and 100.0g of 2,6-dichloroaniline raw materials into the reaction flask, heat and stir in an oil bath to dissolve, stir and reflux at 95°C-100°C for 5h, then cool to room temperature, then cool to 0°C, stir and crystallize for 2h, Suction filtered and dried to obtain 92.0 g of white solid with a yield of 78.4%.
[0047] B. Synthesis of Intermediate 2
[0048]Add 70ml of thionyl chloride and 92g of intermediate 1 into the reaction flask, stir, cool down to 0°C in an ice-water bath, and add a mixed solution of 141ml of thionyl chloride and 156ml of sulfonyl chloride. After the addition, the temperature was naturally raised to room temperature, and then heated to 40°C and stirred for 20 hours. After the system was dissolved, it was distilled under reduced pressure until there was no distillate. Chloromethane solution, after dropping, naturally warm up to room temperature, stop the reaction for 2 hours, and ...
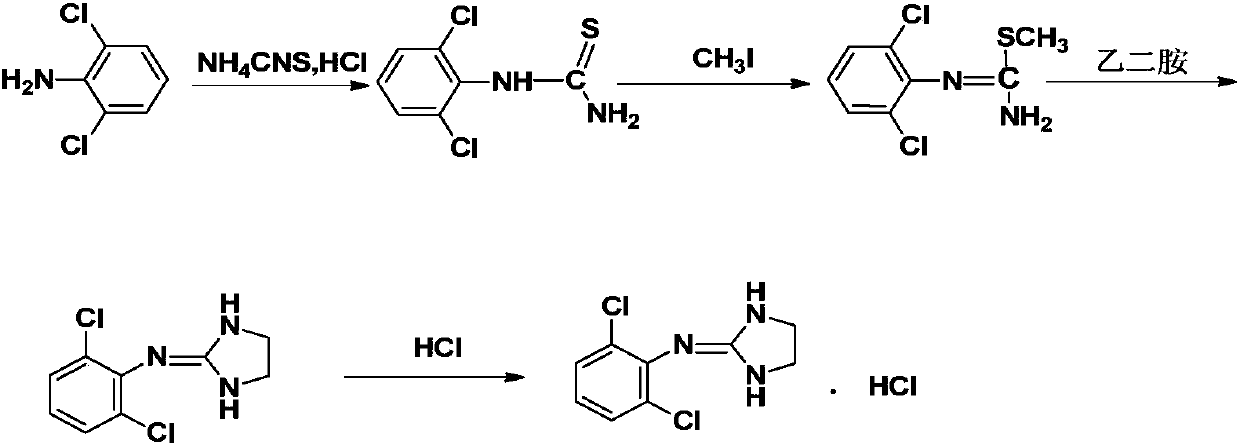
Embodiment 2
[0052] A. Synthesis of Intermediate 1
[0053] Add 200ml of formic acid and 120.0g of 2,6-dichloroaniline raw materials into the reaction flask, heat and stir in an oil bath to dissolve, stir and reflux at 95°C for 6h, then cool to room temperature, then cool to 0°C, stir and crystallize for 2.5h, and filter with suction , and dried to obtain 100.0 g of white solid with a yield of 80.2%.
[0054] B. Synthesis of Intermediate 2
[0055] Add 80ml of thionyl chloride and 100g of intermediate 1 into the reaction flask, stir, cool down to 0°C in an ice-water bath, and add a mixture of 160ml of thionyl chloride and 160ml of sulfuryl chloride. After the addition, the temperature was naturally raised to room temperature, then raised to 45°C and stirred for 18 hours. After the system was dissolved, it was distilled under reduced pressure until there was no distillate. Added 320ml of dichloromethane, cooled to 5°C in an ice-water bath, and added 150g of ethylenediamine distillate dropw...
Embodiment 3
[0059] A. Synthesis of Intermediate 1
[0060] Add 210ml of formic acid and 130.0g of 2,6-dichloroaniline raw materials into the reaction flask, heat and stir in an oil bath to dissolve, stir and reflux at 100°C for 6.5h, then cool to room temperature, then cool to 0°C, stir and crystallize for 3.5h, pump It was filtered and dried to obtain 110 g of white solid with a yield of 82%.
[0061] B. Synthesis of Intermediate 2
[0062] Add 85ml of thionyl chloride and 110g of intermediate 1 into the reaction flask, stir, cool down to 0°C in an ice-water bath, and add a mixture of 150ml of thionyl chloride and 150ml of sulfuryl chloride. After the addition, the temperature was naturally raised to room temperature, then raised to 60°C and stirred for 22 hours. After the system was dissolved, it was distilled under reduced pressure until there was no fraction. Chloromethane solution, after dripping, naturally warmed up to room temperature, and the reaction was stopped for 2 hours. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com