Clonidine hydrochloride oral membrane and preparation method thereof
A technology of clonidine hydrochloride and oral film, which is applied in the direction of anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., can solve the problems of prone to thrombosis and ischemic stroke, and achieve faster drug absorption, Accelerated efficacy, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
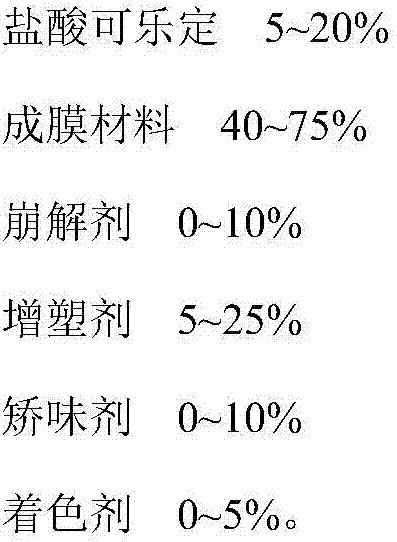
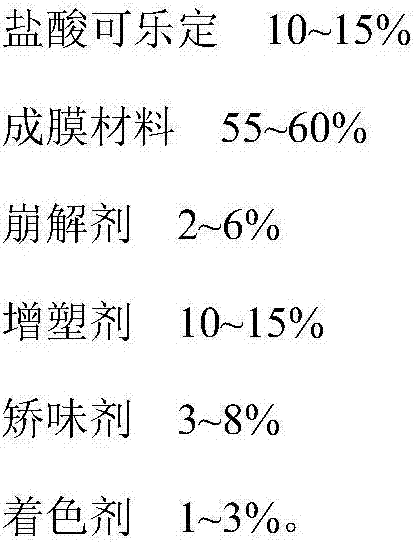
[0042] Clonidine hydrochloride oral film, comprising the following components:
[0043] Clonidine Hydrochloride 20g
[0044] Poloxamer 70g
[0045] Polyethylene glycol 10g.
[0046] The preparation method comprises the following steps:
[0047] (1) Weigh the prescribed amount of poloxamer, add 60°C water to swell, and cool to 50°C when the solution is completely clear;
[0048] (2) Add the polyethylene glycol of recipe quantity, stir constantly to mix, cool to room temperature;
[0049] (3) add the clonidine hydrochloride of recipe quantity, fully stir and make dissolving, and mix homogeneously;
[0050] (4) Stand still for 2 hours, ultrasonic degassing, ultrasonic 10s each time, interval 3s, control temperature at 20-30°C to prepare film material;
[0051] (5) Pour the film material onto the lower edge of the glass plate, push the film material forward with a push rod to coat the film, dry it, remove the film and cut it into required specifications, seal it and pack it, ...
Embodiment 2
[0054] Clonidine hydrochloride oral film, comprising the following components:
[0055] Clonidine Hydrochloride 10g
[0056] Polyvinyl alcohol 80g
[0057] Sodium carboxymethyl starch 5g
[0058] Glycerin 5g.
[0059] The preparation method comprises the following steps:
[0060] (1) Weigh the polyvinyl alcohol of the prescribed amount, add 70°C water to swell, and cool to 50°C when the solution is completely clear;
[0061] (2) Add the sodium carboxymethyl starch and glycerin of recipe quantity, constantly stir to mix, be cooled to room temperature;
[0062] (3) add the clonidine hydrochloride of recipe quantity, fully stir and make dissolving, and mix homogeneously;
[0063] (4) Stand still for 4 hours, ultrasonic degassing, ultrasonic 10s each time, interval 3s, control the temperature and keep it at 20-30°C to prepare the film material;
[0064] (5) Pour the film material onto the lower edge of the glass plate, push the film material forward with a push rod to coat t...
Embodiment 3
[0067] Clonidine hydrochloride oral film, comprising the following components:
[0068] Clonidine Hydrochloride 15g
[0069] Sodium Alginate 60g
[0070] Crosslinked PVP 5g
[0071] Polypropylene glycol 10g
[0072] Sucralose 8g
[0073] Indigo 2g.
[0074] The preparation method comprises the following steps:
[0075] (1) Weigh the sodium alginate of the prescribed amount, add 50°C water to swell, and cool to 30°C when the solution is completely clear;
[0076] (2) Add the cross-linked PVP, polypropylene glycol, sucralose and indigo of the prescription amount, stir continuously until evenly mixed, and cool to room temperature;
[0077] (3) add the clonidine hydrochloride of recipe quantity, fully stir and make dissolving, and mix homogeneously;
[0078] (4) Stand still for 3 hours, ultrasonic degassing, ultrasonic 10s each time, interval 3s, control the temperature and keep it at 20-30°C to prepare the film material;
[0079] (5) Pour the film material onto the lower ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com