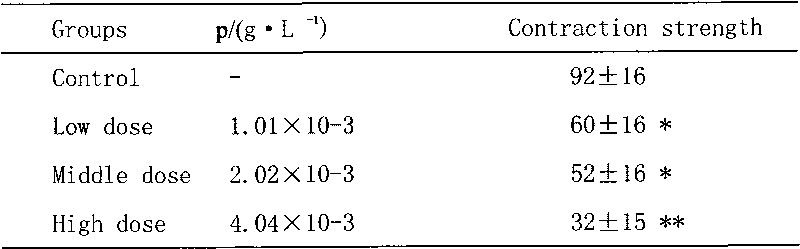External preparation for treating abdominal pain and women's menorrhalgia, preparation method thereof, and quality control method thereof
A technology for external preparations and dysmenorrhea, applied in the field of external preparations, can solve the problems of difficult radical cure, normal morphological and biochemical examinations, and high incidence, and achieve rapid onset of effect, improved bioavailability, and high bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1 gets the raw material of following mass percentage:
[0070] Stearic acid 6.0%
[0071] Stearyl Alcohol 2.0%
[0072] Proe-A100 2.0%
[0073] Isopropyl Palmitate 5.0%
[0074] Propylene Glycol 10.0%
[0075] Glycerin 5.0%
[0076] Imidazolidinyl urea 0.3%
[0077] Carbomer 934P 0.8%
[0078] Clonidine Hydrochloride 0.1%
[0079] Galangal Oil 1.0%
[0080] Cyperus oil 1.0%
[0081] Potassium hydroxide appropriate amount
[0082] Deionized water balance.
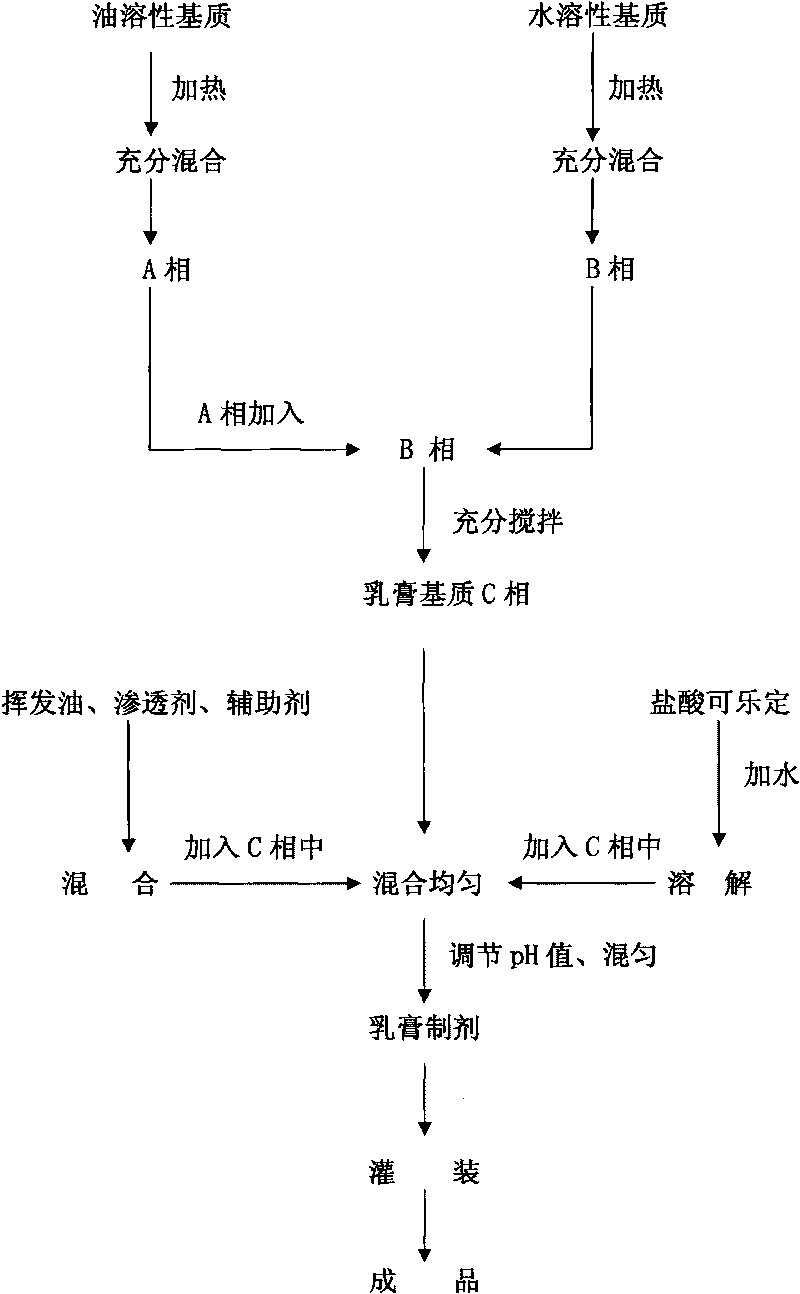
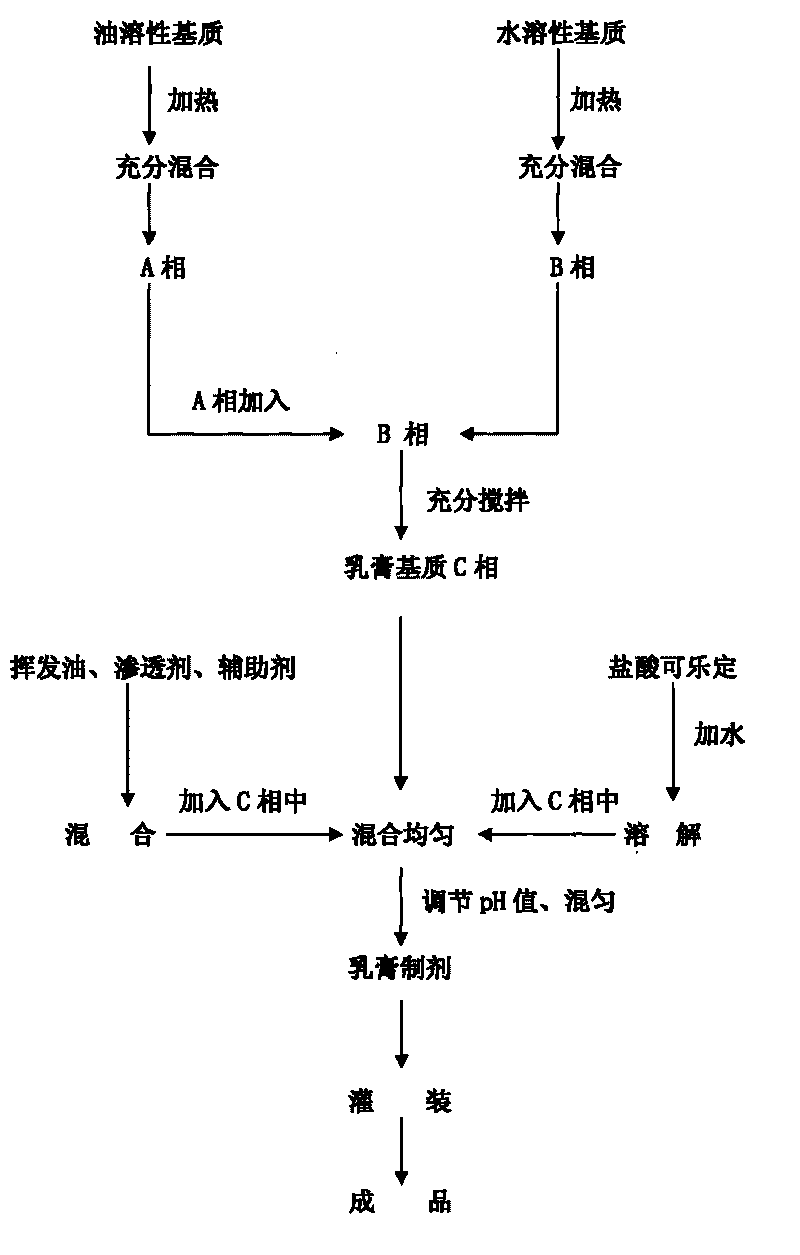
[0083] Preparation method: Mix stearic acid, glycerin, stearyl alcohol, and isopropyl palmitate about 1 / 2 of the weight evenly, heat to 65°C-90°C, and completely dissolve to obtain oil phase A; mix 1 / 2 of the weight Mix Proe-A100, propylene glycol, imidazolidinyl urea, carbomer 934P and part of water and heat to 65℃~90℃ to obtain water phase B; add phase A to phase B, stir to obtain a milky white liquid, stir and cool to obtain a cream Substrate C: Dissolve clonidine hydrochloride with the rest ...
Embodiment 2
[0084] Embodiment 2 gets the raw material of following mass percentage:
[0085] Stearic acid 8.0%
[0086] Stearyl Alcohol 2.0%
[0087] Proe-A100 3.0%
[0088] Isopropyl Palmitate 5.0%
[0089] Imidazolidinyl urea 0.3%
[0090] Propylene Glycol 10%
[0091] Glycerin 5.0%
[0092] Carbomer 934P 0.6%
[0093] Clonidine Hydrochloride 0.1%
[0094] Galangal Oil 1.0%
[0095] Cyperus oil 1.0%
[0096] Appropriate amount of potassium hydroxide
[0097] Purified water balance.
[0098] Preparation method: Mix stearic acid, glycerin, stearyl alcohol, and isopropyl palmitate about 1 / 2 of the weight evenly, heat to 65°C-90°C, and completely dissolve to obtain oil phase A; mix 1 / 2 of the weight Mix Proe-A100, propylene glycol, imidazolidinyl urea, carbomer 934P and part of water and heat to 65℃~90℃ to obtain water phase B; add phase A to phase B, stir to obtain a milky white liquid, stir and cool to obtain a cream Substrate C: Dissolve clonidine hydrochloride with the rest of...
Embodiment 3
[0099] Embodiment 3 gets the raw material of following mass percentage:
[0100] Stearic Acid 4.0%
[0101] Stearyl Alcohol 2.0%
[0102] M68 emulsifying wax 2.0%
[0103] Propylene Glycol 10.0%
[0104] Menthol 0.5%
[0105] Glycerin 5.0%
[0106] Imidazolidinyl urea 0.3%
[0107] Carbomer 940 0.5%
[0108] Clonidine Hydrochloride 0.1%
[0109] Galangal Oil 1.0%
[0110] Cyperus oil 1.0%
[0111] Potassium hydroxide appropriate amount
[0112] Double distilled water remaining.
[0113] Preparation method: Mix stearic acid, glycerin, stearyl alcohol, and M68 emulsifying wax about 1 / 2 of the weight evenly, heat to 65°C-90°C, and completely dissolve to obtain oil phase A; mix 1 / 2 of the weight of Proe- Mix A100, propylene glycol, imidazolidinyl urea, carbomer 934P with some water and heat to 65°C-90°C to obtain water phase B; add phase A to phase B, stir to obtain milky white liquid, stir and cool to obtain cream base C Dissolve clonidine hydrochloride with the water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com