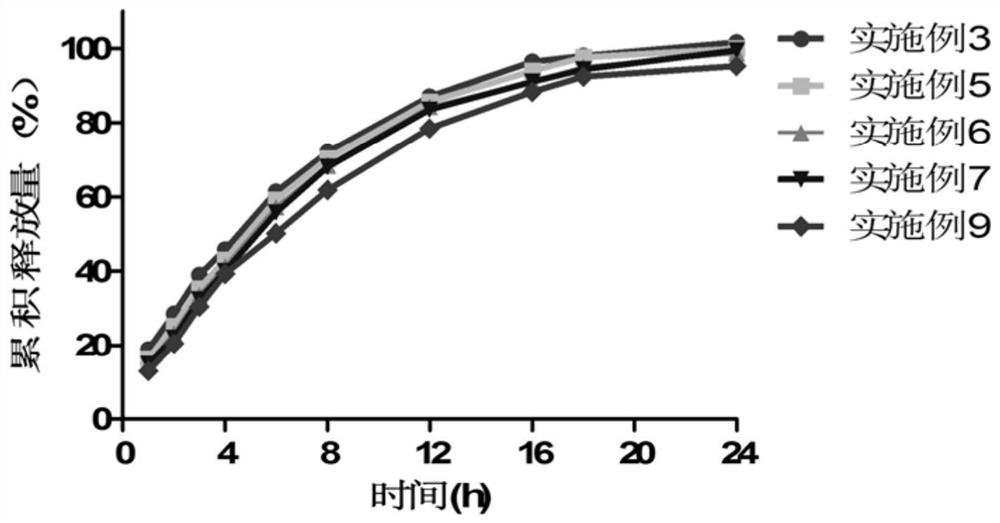
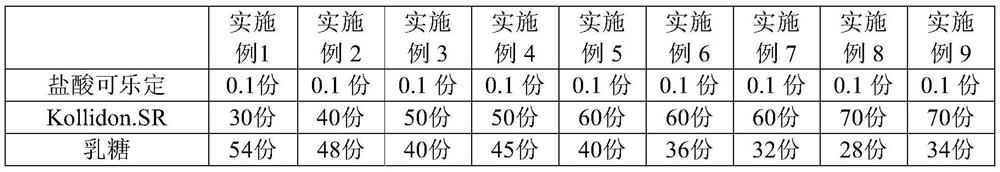
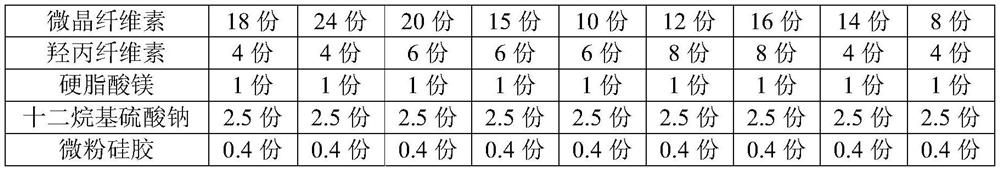
Clonidine hydrochloride sustained-release tablet composition and preparation method thereof
A technology for clonidine hydrochloride and sustained-release tablets, which is applied in drug combinations, pharmaceutical formulas, drug delivery, etc., can solve the problems of poor stability of preparations, high ratio requirements, unfavorable industrial production, etc., and achieve good compressibility and tablet quality. The drug has high hardness and the effect of reducing the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The specific implementation of the present invention will be further described below in conjunction with the examples, but not limited thereto. Based on the present disclosure, those skilled in the art can utilize the present invention to the fullest extent. Therefore, the preferred embodiments described above are illustrative only and do not limit the scope of the present invention in any way.
[0022] A preparation method of clonidine hydrochloride sustained-release tablet, comprising the following steps:
[0023] 1) The clonidine hydrochloride crude drug is pulverized by a pulverizer, passed through a 100-mesh sieve, and set aside;
[0024] 2) Pass Kollidon.SR, microcrystalline cellulose, lactose and hydroxypropyl cellulose through 80-100 mesh sieve respectively;
[0025] 3) Weigh the prescribed amount of clonidine hydrochloride, Kollidon.SR, microcrystalline cellulose, lactose and hydroxypropyl cellulose into the wet granulation pot, turn on the stirring, stir and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com