Clonidine hydrochloride freeze-dried orally disintegrating tablet and preparation method thereof
A technology for cola hydrochloride and dry mouth, applied in the field of medicine, can solve the problems of easy thrombosis, ischemic cerebral apoplexy and the like, and achieve the effects of accelerating drug absorption, facilitating drug dissolution and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
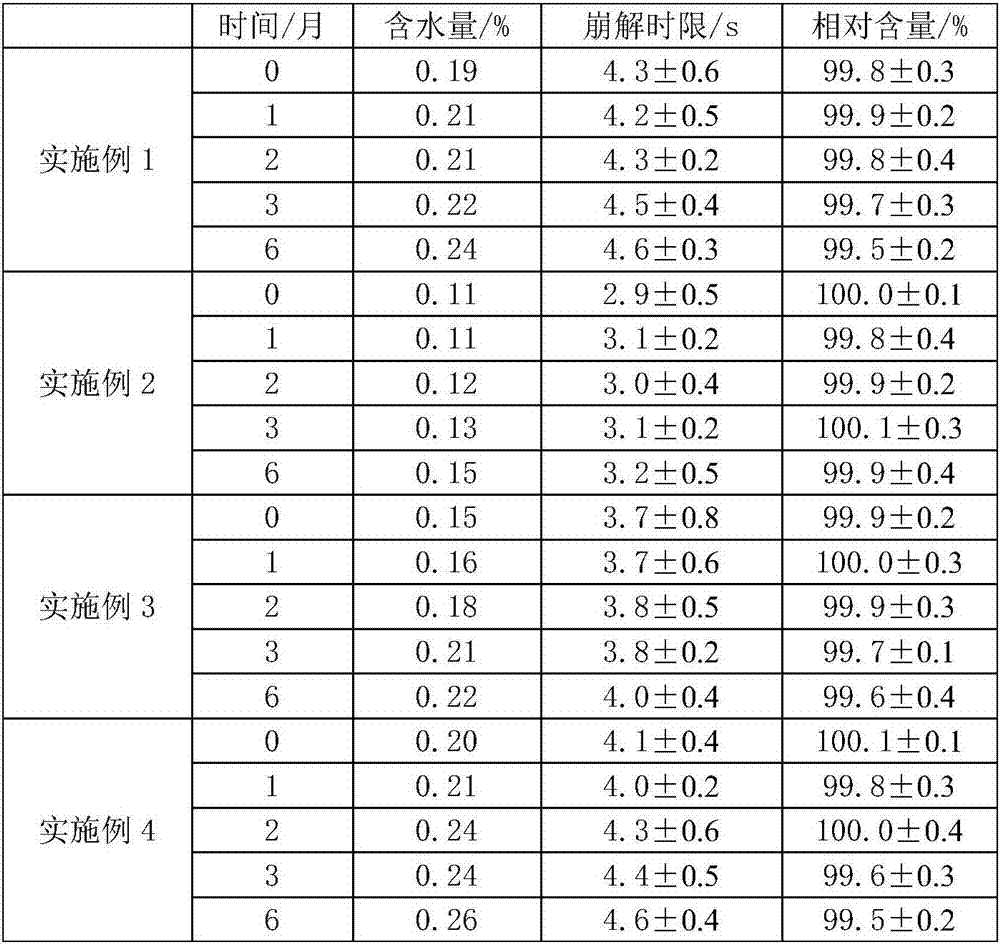
[0028] A clonidine hydrochloride freeze-dried orally disintegrating tablet, comprising: 5 g of clonidine hydrochloride, 90 g of xylitol, and 2 g of gelatin.
[0029] Dissolve the prescribed amount of gelatin in 750mL of purified water and heat until completely dissolved; add the prescribed amount of clonidine hydrochloride and xylitol, stir to dissolve, then add 250mL of purified water to make up the volume; The filling amount is transferred to the mold, and placed in a freeze-drying box, and the liquid medicine is freeze-dried to control the moisture content of not more than 1.0%, sealed, and packaged as a finished product. The specific steps of freeze-vacuum drying are: freeze at -20°C for 4 hours, slowly heat up to 5°C and dry for 12 hours, continue to heat up to 25°C and dry for 10 hours, cool down to 20°C and keep for 4 hours, and the vacuum degree is controlled at 12 Pa.
Embodiment 2
[0031] A clonidine hydrochloride freeze-dried orally disintegrating tablet, the components of which are: 2 g of clonidine hydrochloride, 80 g of sorbitol, 1 g of dextran, an appropriate amount of hydrochloric acid, 1 g of aspartame, and 1 g of methylparaben.
[0032] Dissolve the prescribed amount of dextran in 500mL distilled water and heat until completely dissolved; dissolve the prescribed amount of clonidine hydrochloride and sorbitol in the above solution, add the prescribed amount of aspartame and methyl paraben, and stir uniform; use hydrochloric acid to adjust the pH value to 3, and use 500mL distilled water to fix the volume; after determining the filling amount according to the specifications, transfer the liquid medicine to the mold according to the filling volume, and place it in a freeze-drying box to freeze and vacuum-dry the liquid medicine to control The water content does not exceed 1.0%, and it is sealed and packaged as a finished product. The specific steps ...
Embodiment 3
[0034] A clonidine hydrochloride freeze-dried orally disintegrating tablet, the components of which are: 10 g of clonidine hydrochloride, 98 g of mannitol, 5 g of sodium alginate, an appropriate amount of hydrochloric acid, 1 g of sucralose, and 0.5 g of sodium benzoate.
[0035] Dissolve the prescribed amount of sodium alginate in 600mL deionized water, and heat until completely dissolved; dissolve the prescribed amount of clonidine hydrochloride and mannitol in the above solution, add the prescribed amount of sucralose and sodium benzoate, and stir well; Adjust the pH value to 2.5 with hydrochloric acid, and use 400mL deionized water to make up the volume; after determining the filling volume according to the specifications, transfer the medicine liquid to the mold according to the filling volume, and place it in a freeze-drying box to freeze and vacuum-dry the medicine liquid to control the moisture content. The content is not more than 1.0%; it is sealed and packaged as a f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com