Transdermal patch of clonidine hydrochloride and preparation method thereof
A technology of clonidine hydrochloride and transdermal patch, which is applied in anti-inflammatory agents, medical equipment, and pharmaceutical formulas, and can solve problems such as permeability and unsatisfactory administration time, so as to improve the smoothness of medication, optimize the course of medication, The effect of strengthening crystal growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
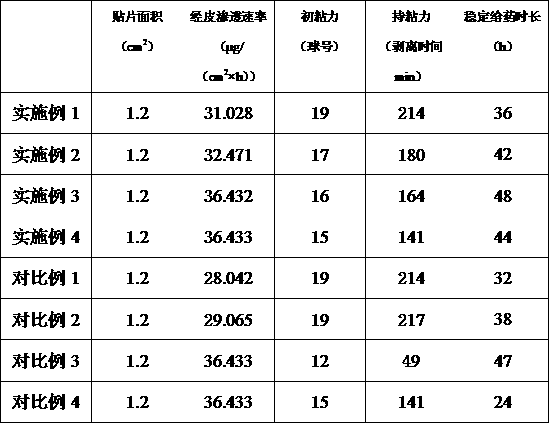
Embodiment 1
[0029] A transdermal patch of clonidine hydrochloride, the patch includes a backing layer and a paste layer, the backing layer includes an identification code with a unique code, and the identification code includes a product display information module, a user text And / or photo information entry module, the paste layer includes 7wt% clonidine hydrochloride, 4wt% triethylamine, 4wt% diethanolamine, 0.3wt% antioxidant 1010, 64wt% TPE-S resin , 20wt% terpene resin. The preparation method of aforementioned transdermal patch, described preparation method comprises the steps:
[0030] 1) Prepare the raw materials of each component according to the aforementioned paste components, dissolve TPE-S resin and terpene resin in acetone, and add clonidine hydrochloride, triethylamine, diethanolamine and antioxidant 1010 to the solution to Ultrasonic treatment with 1200w power for 30min.
[0031] 2) Treat the solution obtained in step 1) with a power of 200w for 5 minutes, apply the mixed ...
Embodiment 2
[0034] A transdermal patch of clonidine hydrochloride, the patch includes a backing layer and a paste layer, the backing layer includes an identification code with a unique code, and the identification code includes a product display information module, a user text And / or photo information entry module, the paste layer includes 10wt% clonidine hydrochloride, 4wt% triethylamine, 5.2wt% diethanolamine, 0.5wt% vitamin E, 60wt% TPE-S resin, 20 wt% terpene resin. The preparation method of aforementioned transdermal patch, described preparation method comprises the steps:
[0035] 1) Prepare the raw materials of each component according to the aforementioned paste components, dissolve TPE-S resin and terpene resin in acetone, add clonidine hydrochloride, triethylamine, diethanolamine and antioxidant to the solution respectively, and use 1000w Ultrasonic treatment with high power for 45min.
[0036] 2) Treat the solution obtained in step 1) with a power of 300w for 3 minutes, apply...
Embodiment 3
[0039] A transdermal patch of clonidine hydrochloride, the patch includes a backing layer and a paste layer, the backing layer includes an identification code with a unique code, and the identification code includes a product display information module, a user text And / or photo information entry module, the paste layer includes 15wt% clonidine hydrochloride, 5wt% triethylamine, 6.5wt% diethanolamine, 0.6wt% vitamin E, 58wt% TPE-S resin, 14.9 wt% terpene resin. The preparation method of aforementioned transdermal patch, described preparation method comprises the steps:
[0040] 1) Prepare the raw materials of each component according to the aforementioned paste components, dissolve TPE-S resin and terpene resin in acetone, add clonidine hydrochloride, triethylamine, diethanolamine and antioxidant to the solution respectively, and use 1100w Ultrasonic treatment with high power for 40min.
[0041] 2) Treat the solution obtained in step 1) with a power of 300w for 3 minutes, app...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com