Preparation method of vildagliptin and metformin hydrochloride compound preparation
A technology of metformin hydrochloride and compound preparations, which is applied in the directions of pill delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of large disparity in dose ratio, poor fluidity, and increased impurity content, and achieves production equipment. The effect of low cost, simple process operation and little impurity change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
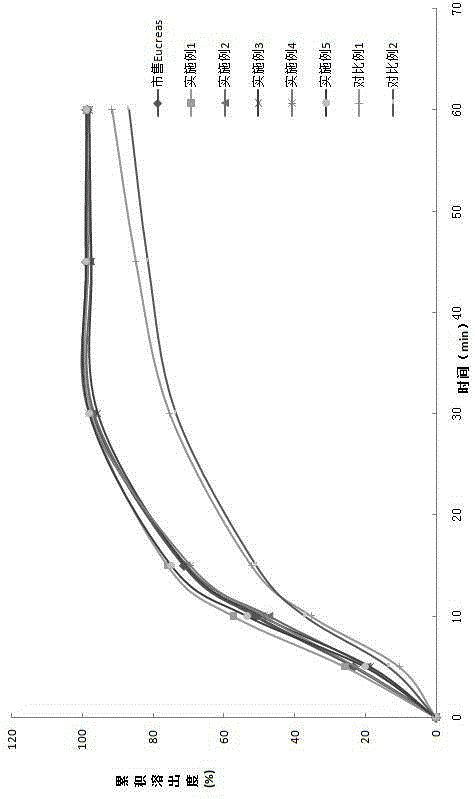
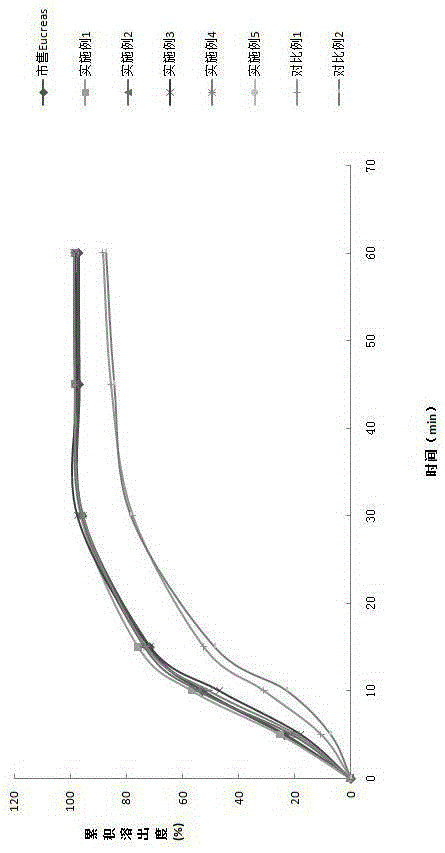
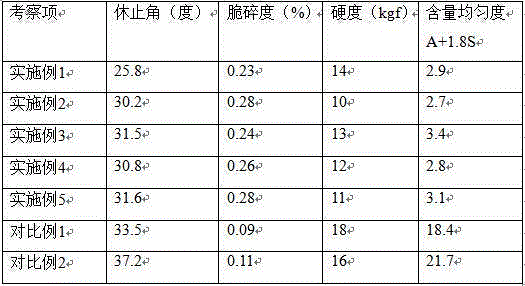
Image
Examples
Embodiment 1
[0061] 95% ethanol wet granulation
[0062]
[0063] The preparation process is as follows:
[0064] (1) Take vildagliptin, metformin hydrochloride and hydroxypropyl cellulose SL of prescription quantity and pass through 60 mesh sieves respectively, mix evenly;
[0065] (2) Use 95% ethanol solution (200 mL) to make a soft material, granulate with a 20-mesh sieve, and dry at 60°C;
[0066] (3) Mix the granules in step (2) with magnesium stearate, take a sample for testing, calculate the tablet weight, and compress the tablet.
Embodiment 2
[0068] dry granulation
[0069]
[0070] Its preparation process is as follows:
[0071] (1) Take vildagliptin, metformin hydrochloride and hydroxypropyl cellulose SL-FP of the prescribed amount by weighing and pass through a 60-mesh sieve respectively, and mix well;
[0072] (2) Put the mixture of step (1) into the dry granulator, and the granulation parameters are set as follows: the distance between the pinch wheels is 0.3 mm, the speed of the pinch wheels is 2~4 rpm, the feeding speed is 20~30 rpm, and the granulation is done through a 20-mesh sieve. , and sifted to 80 mesh fine powder;
[0073] (3) Mix the granules in step (2) with magnesium stearate, take a sample for testing, calculate the tablet weight, and compress the tablet.
Embodiment 3
[0075] dry granulation
[0076]
[0077] Its preparation process is as follows:
[0078] (1) Take vildagliptin, metformin hydrochloride and hydroxypropyl cellulose SSL-SFP of the prescribed amount by weighing and pass through a 60-mesh sieve respectively, and mix well;
[0079] (2) Put the mixture in step (1) into the dry granulator, and the granulation parameters are set as follows: pitch between pinch wheels 0.2 mm, speed of pinch wheels 3~5 rpm, speed of feeding 40~50 rpm, granulation with 20-mesh sieve , and sifted to 80 mesh fine powder;
[0080] (3) Mix the granules in step (2) with magnesium stearate, take a sample for testing, calculate the tablet weight, and compress the tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com