Ticagrelor tablets and preparation method thereof
A technology of ticagrelor and mannitol, which is applied in the field of medicine, can solve the problems affecting bioavailability and curative effect, affecting the full absorption of drugs, and uneven dissolution, so as to improve bioavailability, easy operation and realization, and dissolution rate fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
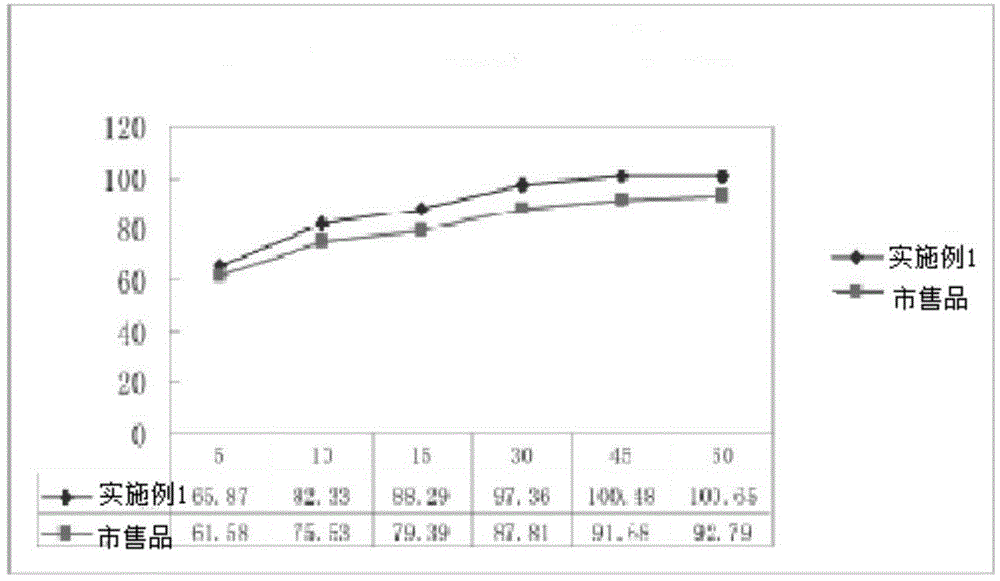
Embodiment 1
[0045] The present embodiment ticagrelor tablet is made up of the following components in percentage by weight: ticagrelor 29.1%, mannitol 40.74%, microcrystalline cellulose 20.37%, low-substituted hydroxypropyl cellulose 2.91%, alternating Bihydroxymethylcellulose sodium 2.91%, magnesium stearate 0.97%, film coating premix 3%.
[0046] Among them, the film coating premix was purchased from Shanghai Colorcon Coating Technology Co., Ltd., and the product number was 295B620026.
[0047] The present embodiment prepares 30000 ticagrelor tablets, and its preparation method is: the specific operation steps are as follows:
[0048] 1) Weigh 2.7kg of ticagrelor tablets, 3.78kg of mannitol, 1.89kg of microcrystalline cellulose, 0.27kg of low-substituted hydroxypropyl cellulose, and 0.135kg of croscarmellose sodium, and pass through 100 meshes respectively. After sieving, place in a wet mixing granulator and mix for 3 minutes to obtain a mixed powder;
[0049] 2) Add 2.8 kg of purifie...
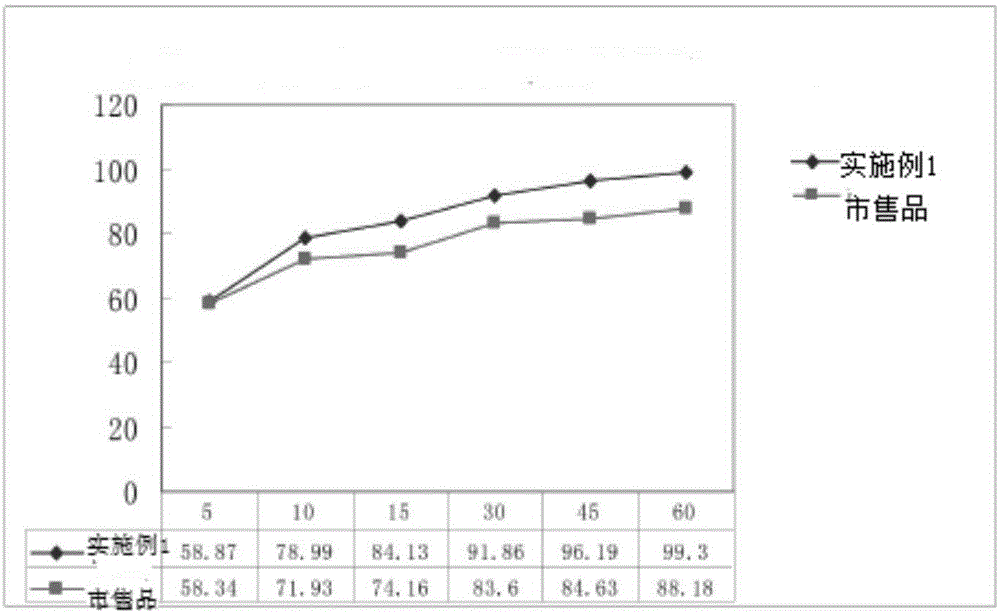
Embodiment 2
[0055] The present embodiment ticagrelor tablet is made up of the following components in percentage by weight: ticagrelor 29.55%, mannitol 41.37%, microcrystalline cellulose 20.69%, low-substituted hydroxypropyl cellulose 2.96%, alternating Bihydroxymethylcellulose sodium 2.96%, magnesium stearate 0.99%, film coating premix 1.5%.
[0056] Among them, the film coating premix was purchased from Shanghai Colorcon Coating Technology Co., Ltd., and the product number was 295B620026.
[0057] The present embodiment prepares 30000 ticagrelor tablets, and its preparation method is: the specific operation steps are as follows:
[0058] 1) Weigh 2.7kg of ticagrelor tablets, 3.78kg of mannitol, 1.89kg of microcrystalline cellulose, 0.27kg of low-substituted hydroxypropyl cellulose, and 0.135kg of croscarmellose sodium, and pass through 100 meshes respectively. After sieving, place it in a wet mixing granulator and mix for 5 minutes to obtain a mixed powder;
[0059] 2) Add 3.0 kg of p...
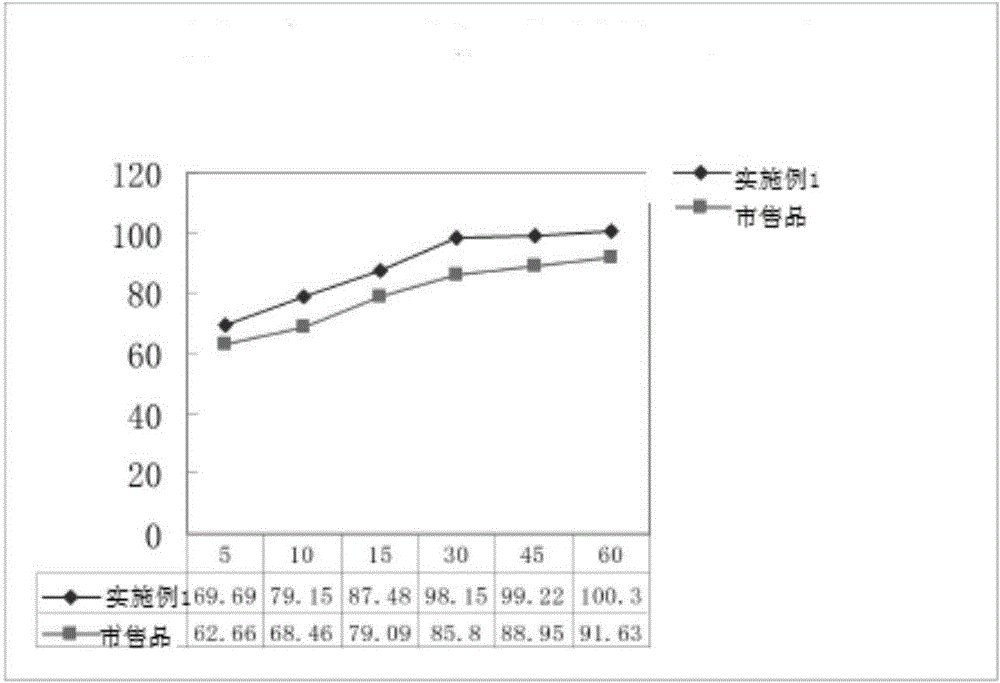
Embodiment 3
[0065] The present embodiment ticagrelor tablet is made up of the following components in percentage by weight: ticagrelor 29.32%, mannitol 41.10%, microcrystalline cellulose 20.54%, low-substituted hydroxypropyl cellulose 2.93%, alternating Bihydroxymethylcellulose sodium 2.93%, magnesium stearate 0.98%, film coating premix 2.2%.
[0066] Among them, the film coating premix was purchased from Shanghai Colorcon Coating Technology Co., Ltd., and the product number was 295B620026.
[0067] The present embodiment prepares 30000 ticagrelor tablets, and its preparation method is: the specific operation steps are as follows:
[0068] 1) Weigh 2.7kg of ticagrelor tablets, 3.78kg of mannitol, 1.89kg of microcrystalline cellulose, 0.27kg of low-substituted hydroxypropyl cellulose, and 0.135kg of croscarmellose sodium, and pass through 100 meshes respectively. After sieving, place in a wet mixing granulator and mix for 4 minutes to obtain a mixed powder;
[0069] 2) Add 2.9 kg of puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com