Benzene sulfonic acid levo-amlodipine pill and preparation method thereof
A kind of technology of levamlodipine besylate and levamlodipine besylate, applied in the field of levamlodipine besylate tablet and preparation thereof, can solve the problems such as no document discloses levamlodipine besylate tablet and the like, achieves Good product stability, good practicability, and improved dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
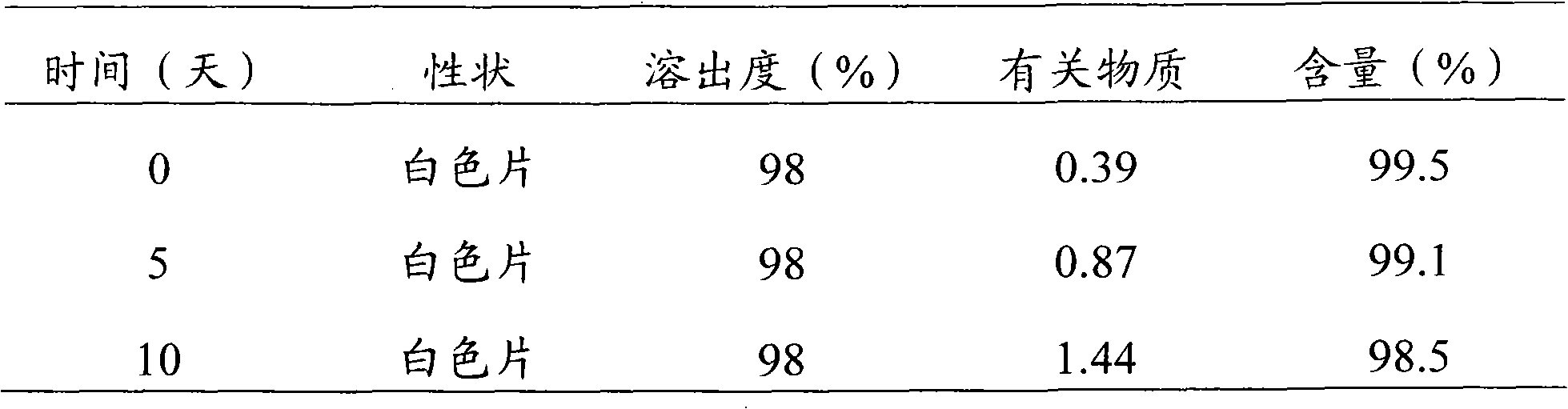
Embodiment 1
[0027] Levoamlodipine Besylate 2.5g (calculated as Levoamlodipine)
[0028] Lactose 80g
[0029] Low-substituted hydroxypropyl cellulose 30g
[0030] Cross-linked polyvinylpyrrolidone 5g
[0031] Magnesium Stearate 1.5g
[0032]
[0033] Press 1000 tablets (theoretical tablet weight 0.12g tablet)
[0034] Preparation:
[0035] (1) According to the above formula, weigh the raw materials and auxiliary materials, mix them by double dispersion and dilution method, and put them in a high-speed ultrafine pulverizer for 1-2 minutes, so that the pulverized mixed powder can pass through a sieve of more than 100 mesh. Obtain uniform mixed fine powder for subsequent use.
[0036] (2) Use ethanol aqueous solution as a wetting agent.
[0037] (3) Stir the wetting agent to the mixed fine powder under each prescription and mix evenly, and make a soft material, use a 16-18 mesh sieve to make granules, and dry in a 60°C oven for 3-4 h...
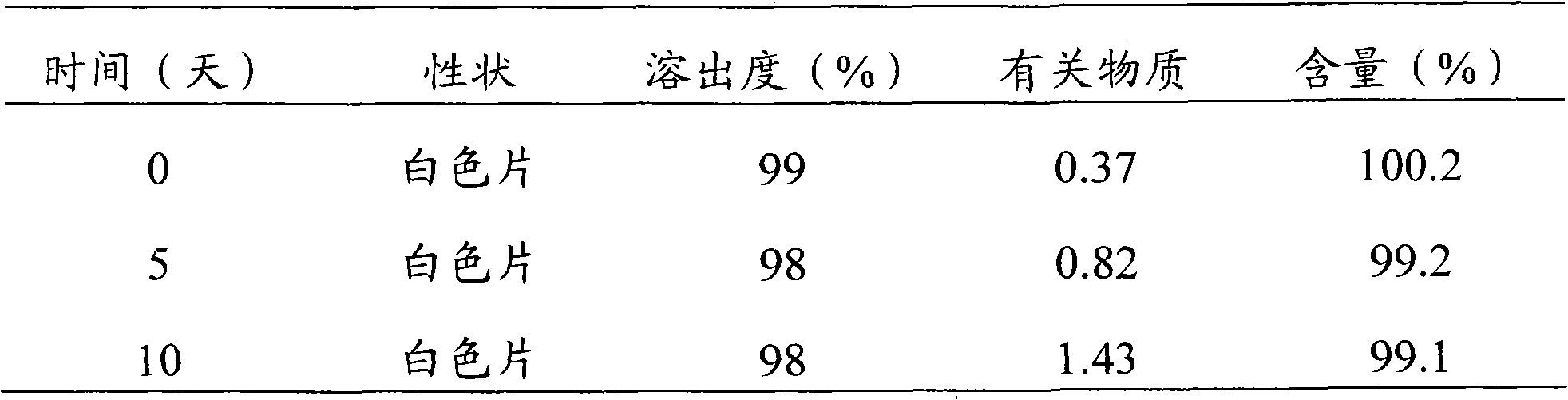
Embodiment 2
[0040] Levoamlodipine Besylate 5.0g (calculated as Levoamlodipine)
[0041] Lactose 80g
[0042] Low-substituted hydroxypropyl cellulose 26.5g
[0043] Cross-linked polyvinylpyrrolidone 5g
[0045]
[0046] Press 1000 tablets (theoretical tablet weight 0.12g tablet)
[0047] Preparation:
[0048] (1) According to the above prescription formula, weigh the raw materials and auxiliary materials (except external auxiliary materials) and mix them by double dispersion and dilution method, and put them in a high-speed ultra-fine pulverizer for 1-2 minutes, so that the pulverized mixed powder can pass through 100 mesh above the sieve. Obtain uniform mixed fine powder for subsequent use.
[0049] (2) Use ethanol aqueous solution as a wetting agent.
[0050] (3) Stir the wetting agent to the mixed fine powder under each prescription and mix evenly, and make a soft material, use a 1...
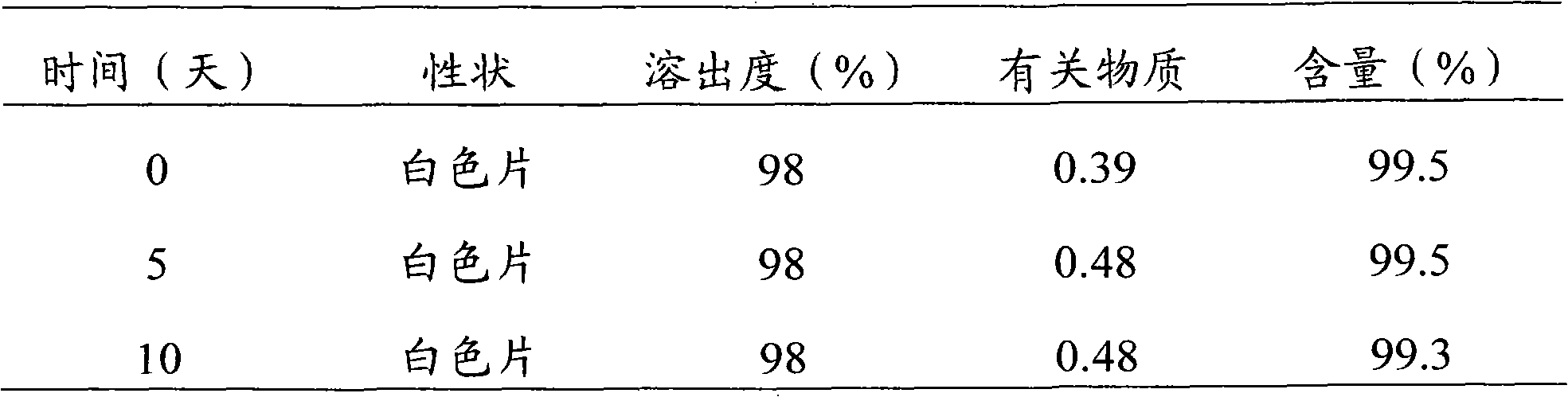
Embodiment 3
[0053] Levoamlodipine Besylate 2.5g (calculated as Levoamlodipine)
[0054] Lactose 70g
[0055] Low-substituted hydroxypropyl cellulose 37g
[0056] Cross-linked polyvinylpyrrolidone 8g
[0057] Magnesium stearate (additional) 1.5g
[0058]
[0059] Press 1000 tablets (theoretical tablet weight 0.12g tablet)
[0060] Preparation:
[0061] (1) According to the above prescription formula, weigh the raw materials and auxiliary materials (except external auxiliary materials) and mix them by double dispersion and dilution method, and put them in a high-speed ultra-fine pulverizer for 1-2 minutes, so that the pulverized mixed powder can pass through 100 mesh above the sieve. Obtain uniform mixed fine powder for subsequent use.
[0062] (2) Use ethanol aqueous solution as a wetting agent.
[0063] (3) Stir the wetting agent to the mixed fine powder under each prescription and mix evenly, and make a soft material, use a 16-18 me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com