Berberine hydrochloride tablet and preparation method thereof
A technology of berberine hydrochloride and tablet cores, which is applied in the fields of pharmaceutical formulas, medical preparations containing active ingredients, metabolic diseases, etc. It can solve the problems that the dissolution rate and bioavailability cannot meet the pharmacopoeia standards, the blood drug concentration cannot be maintained, and the patient Poor compliance and other problems, to achieve the effect of good particle fluidity and forming, not easy to wear, and controllable tablet weight difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] A berberine hydrochloride tablet is composed of a tablet core and a coating material. The tablet core is composed of berberine hydrochloride and auxiliary materials. Each tablet contains 300 mg of berberine hydrochloride. The weight percentage of berberine hydrochloride and auxiliary materials is: small amount of hydrochloric acid Berberine 64-96%, the remaining auxiliary materials 4-36%; the auxiliary materials are one or more of fillers, disintegrants, lubricants, surfactants, and wetting agents; the coating layer is composed of coating materials and water; the composition of the main drug and the auxiliary materials in the tablet core is in parts by weight: berberine hydrochloride 72-88%, filler 5-15%, disintegrant 1-8%, lubricant 0.5- 2%, 0.05-0.8% surfactant, appropriate amount of wetting agent; the coating layer also contains plasticizer or anti-sticking agent, and the weight percentage of each solid component is: coating material 75-100%, plasticizer 0- 20%, anti...
Embodiment 1
[0045] Take berberine hydrochloride 300g, starch 20g, microcrystalline cellulose 25g, crospovidone 15g, Tween-800.8g, magnesium stearate 4g, appropriate amount of 2% hypromellose, premix and wet granulate , drying, granulation, blending, tableting, coating layer ingredients are: Opadry 18g, triethyl citrate 1.5g, glyceryl monostearate 0.3g, Tween-88 0.2g, solvent is water.
[0046] According to the above formula, firstly, the raw and auxiliary materials are premixed and wet granulated; the granules prepared by the wet method are dried and granulated; the obtained dry granules are mixed with additional disintegrants and lubricants; the mixed granules are compressed into tablets; coating .
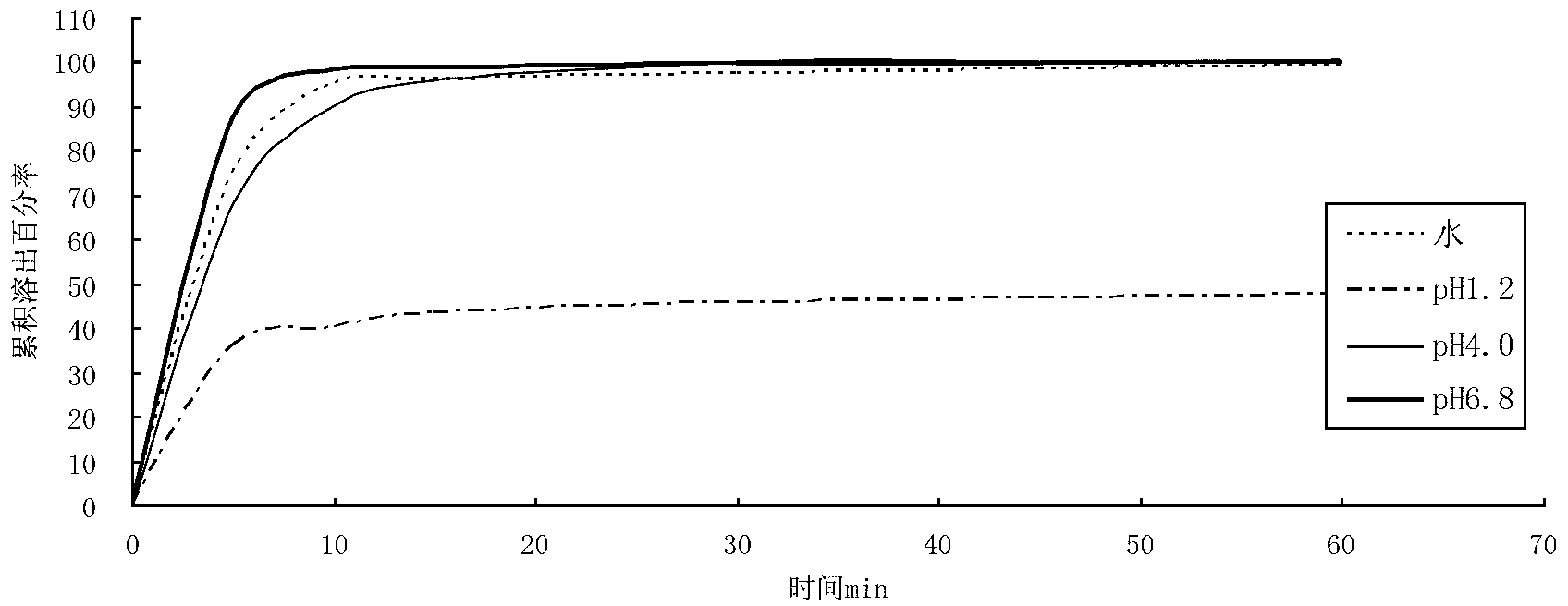
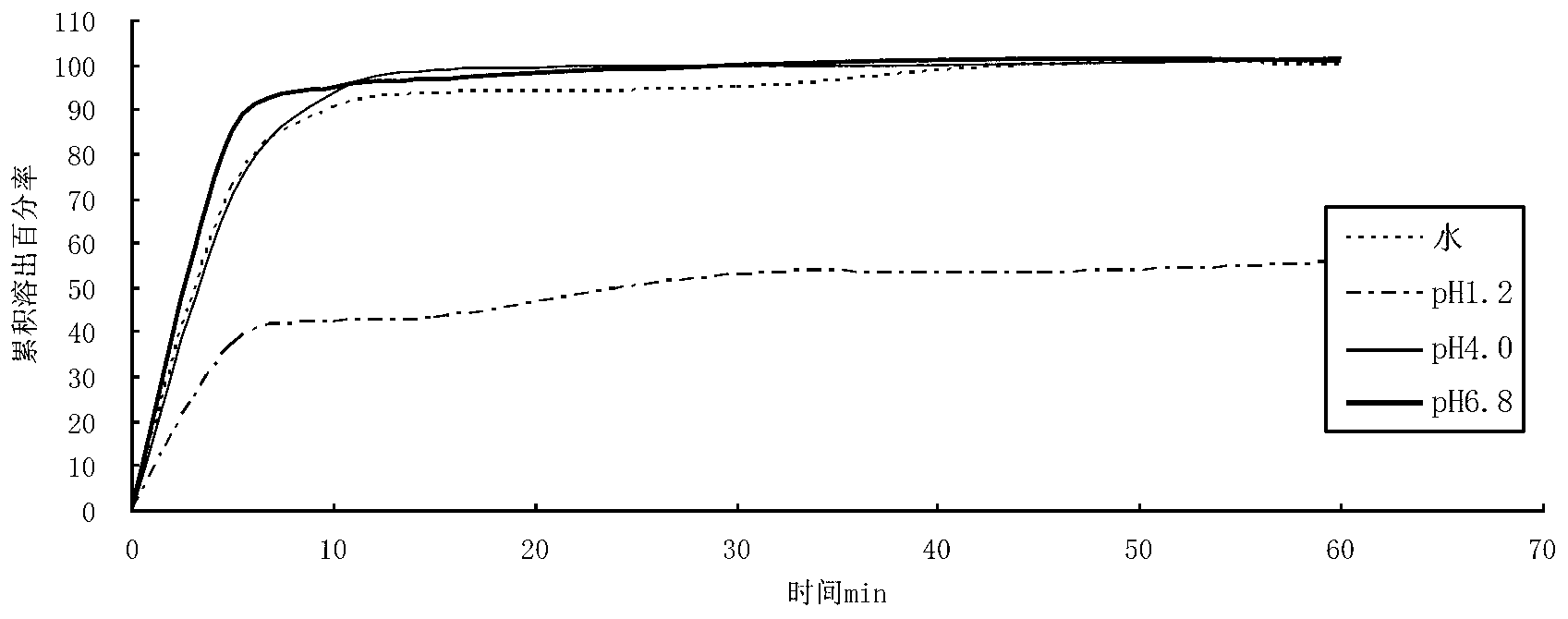
[0047] The berberine hydrochloride tablet made above was carried out dissolution test under different conditions, and the results are shown in the table below.
[0048]
Embodiment 2
[0050] Take 300g of berberine hydrochloride, 10g of lactose, 25g of pregelatinized starch, 24g of sodium carboxymethyl starch, Tween-801g, 5g of micropowdered silica gel, and an appropriate amount of 40% ethanol solution, premix and wet granulate, dry, granulate, Blending and tableting, the coating layer ingredients are: 18.7g of Opadry, 1.0g of triethyl citrate, 0.2g of glyceryl monostearate, 0.1g of Tween-88, and the solvent is water.
[0051] According to the above formula, firstly, the raw and auxiliary materials are premixed and wet granulated; the granules prepared by the wet method are dried and granulated; the obtained dry granules are mixed with additional disintegrants and lubricants; the mixed granules are compressed into tablets; coating .
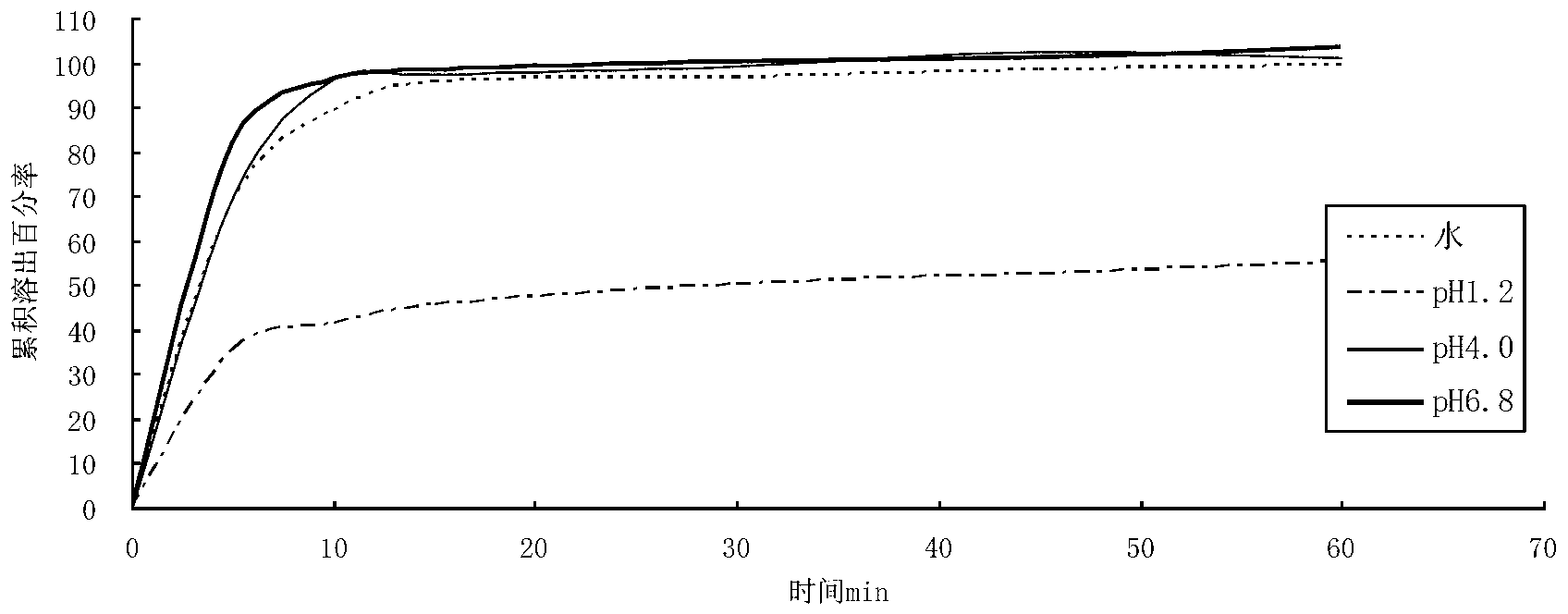
[0052] The berberine hydrochloride tablet made above was carried out dissolution test under different conditions, and the results are shown in the table below.
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com