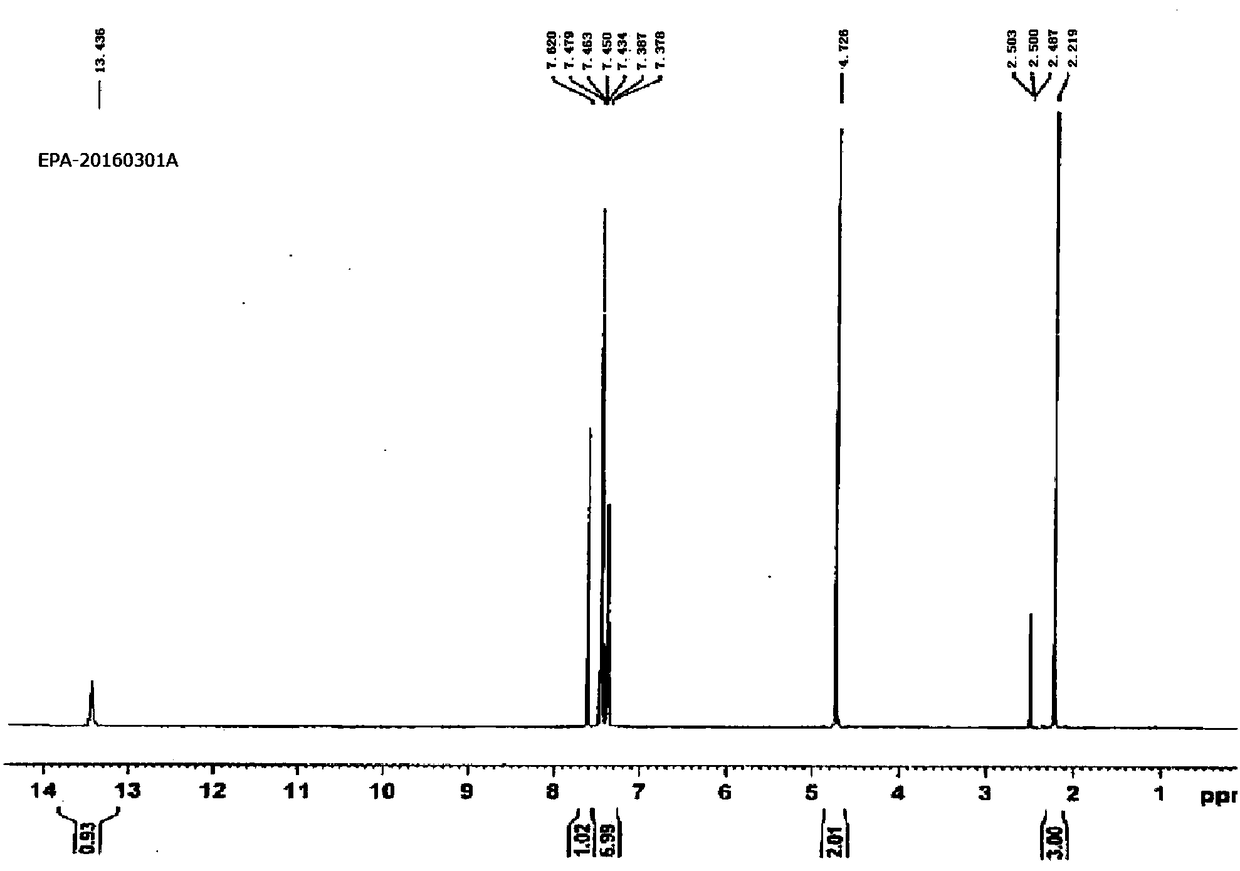
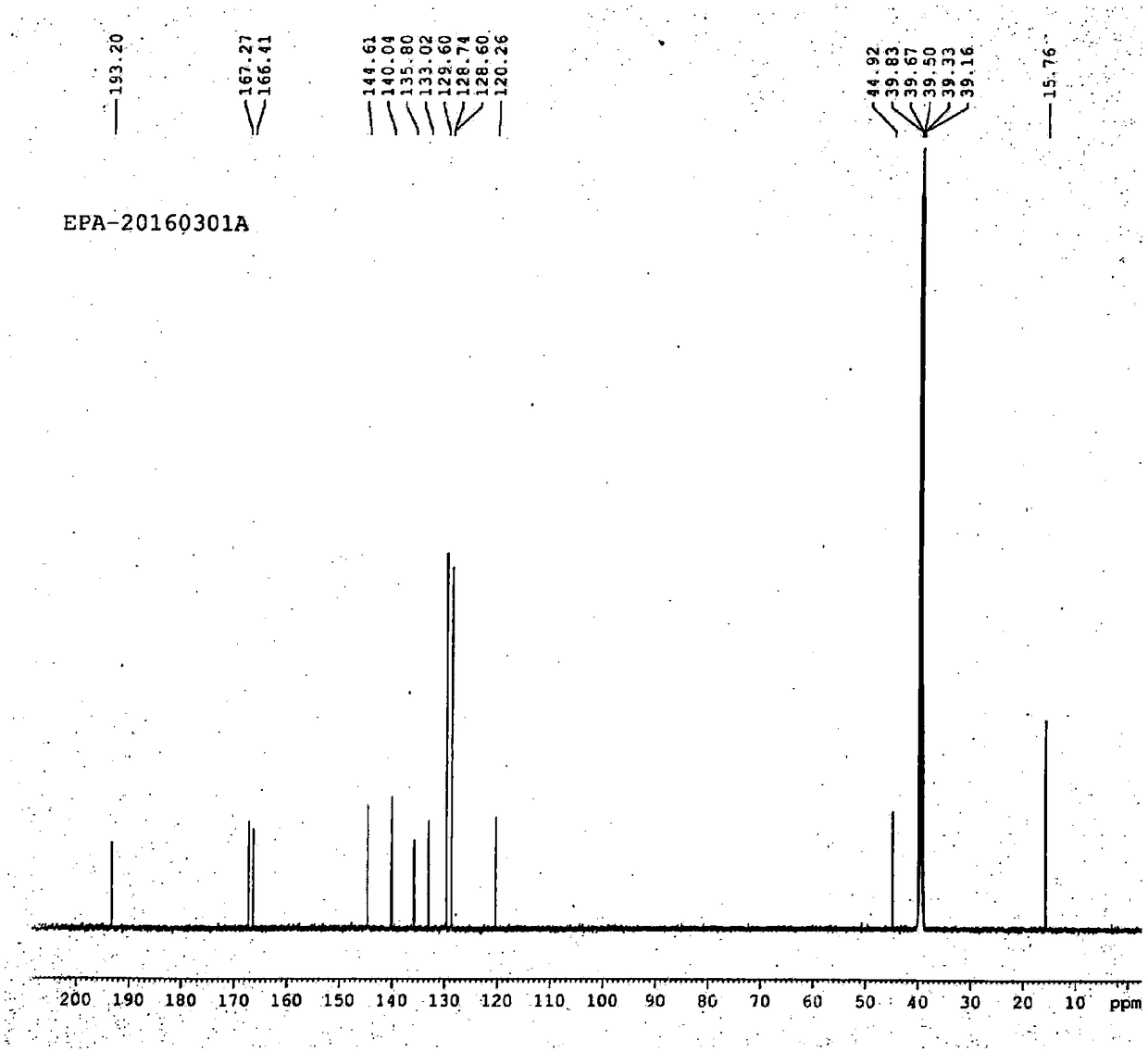
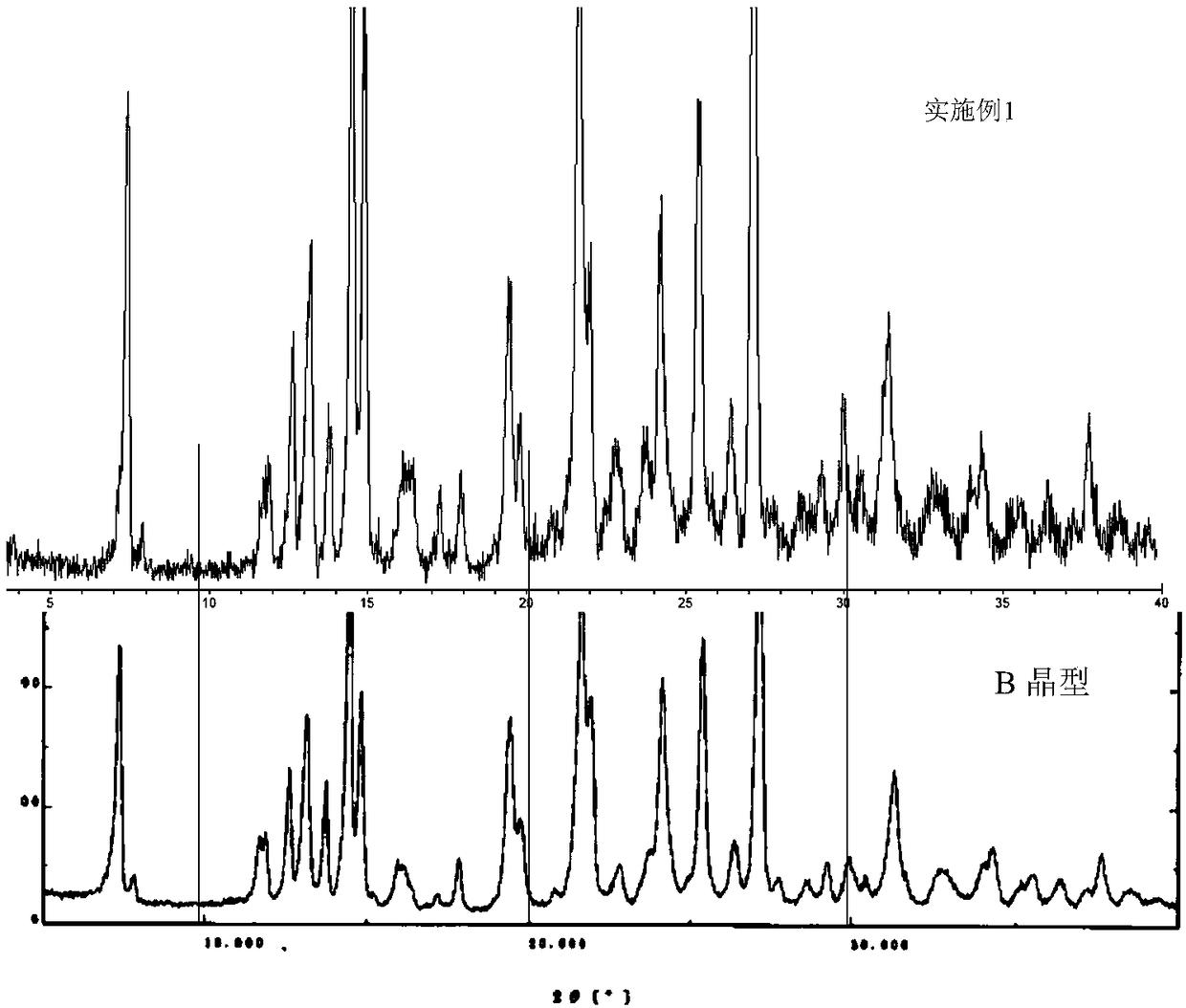
B crystal form epalrestat and preparation method thereof
A technology of epalrestat and crystal form, applied in the field of preparation of crystal form B epalrestat, can solve the problems of long reaction time, complicated operation, poor product purity of crystal form B epalrestat, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] For illustrating epalrestat of the present invention and its synthetic method
[0036] Step 1: The crude product preparation process, add 10L ethanol to the 20L reaction kettle, add 1kg (5.229mol) rhodanine acetic acid, add 1.22L (7.844mol) ammonia water, stir for 18min, add 946ml (6.798mol) α-methyl For cinnamaldehyde, heat up to 70°C, keep warm for 30 minutes, monitor the reaction with TCL (dichloromethane: methanol: acetic acid = 10:1:0.1), the spots of the reactant disappear, cool down to 25°C, and the crude product is not separated.
[0037] Step 2: In the preparation process of the crude product, directly add 1.31 L of concentrated hydrochloric acid (calculated as 1.0 moles of rhodanine acetic acid, and the relative mole number of HCl is 3.0) to the aforementioned crude product, raise the temperature to 50°C, stir for 2 hours, centrifuge, and purify water The filter cake was washed (until the pH of the cleaning waste liquid was 6), and dried to obtain 1078 g of cr...
Embodiment 2
[0044] For illustrating epalrestat of the present invention and its synthetic method
[0045] Step 1: The crude product preparation process, with reference to Example 1, the solvent is changed from ethanol to methanol, the amount of α-methylcinnamaldehyde added is changed from 1.3 to 1.0 in relative moles (calculated with the moles of rhodanine acetic acid being 1.0), the type of alkali From ammonia water to triethylamine, the reaction time is 40min.
[0046] Step 2: The preparation process of the crude fine product, referring to Example 1, the acid type is changed from hydrochloric acid to sulfuric acid, the amount of sulfuric acid added is changed from 3.0 to 4.0 relative moles (calculated as 1.0 moles of rhodanine acetic acid), and the reaction temperature is changed from 50°C Changed to 55°C, the yield was 63wt%.
[0047] Step 3: the finished product preparation process, add 10 times of ethanol 10L (calculated based on the mass of the crude product) to the 20L reactor, ad...
Embodiment 3
[0050] For illustrating epalrestat of the present invention and its synthetic method
[0051] Step 1: The crude product preparation process, with reference to Example 1, the amount of α-methylcinnamaldehyde added is changed from 1.3 to 1.1 in relative moles (calculated based on 1.0 moles of rhodanine acetic acid), and the type of alkali is changed from ammonia to diisopropylamine , The reaction time is 30min.
[0052] Step 2: the preparation process of the crude fine product, with reference to Example 1, the acid type is changed from hydrochloric acid to acetic acid, the amount of acetic acid added is changed from 3.0 to 4.5 relative moles (calculated with rhodanine acetic acid moles as 1.0), and the yield is 67wt% .
[0053] Step 3: the finished product preparation process, add 10 times of methanol 10L (calculated on the basis of the mass of the crude product) in the 20L reactor, add 1 kg of the crude product (prepared in step 2), at room temperature, first drop 732ml of amm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com