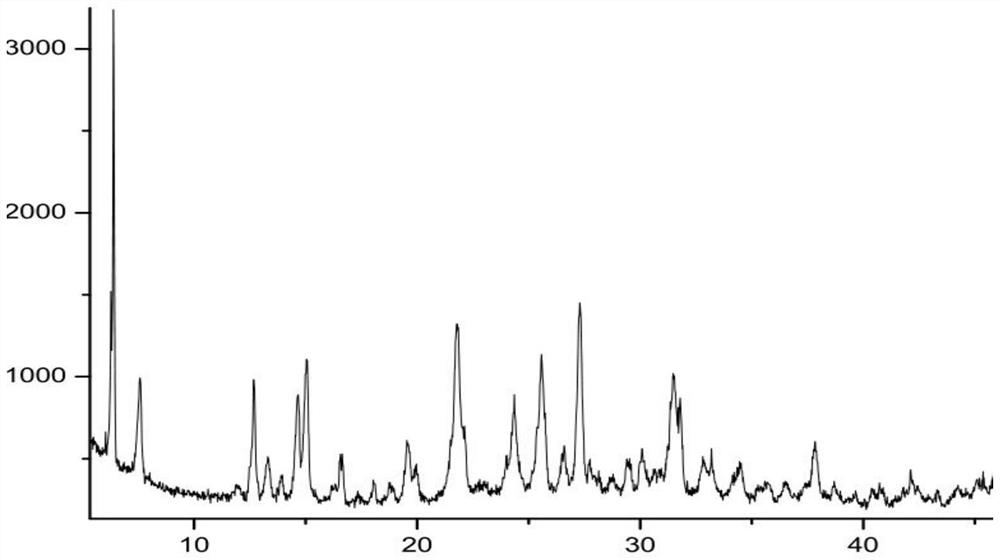
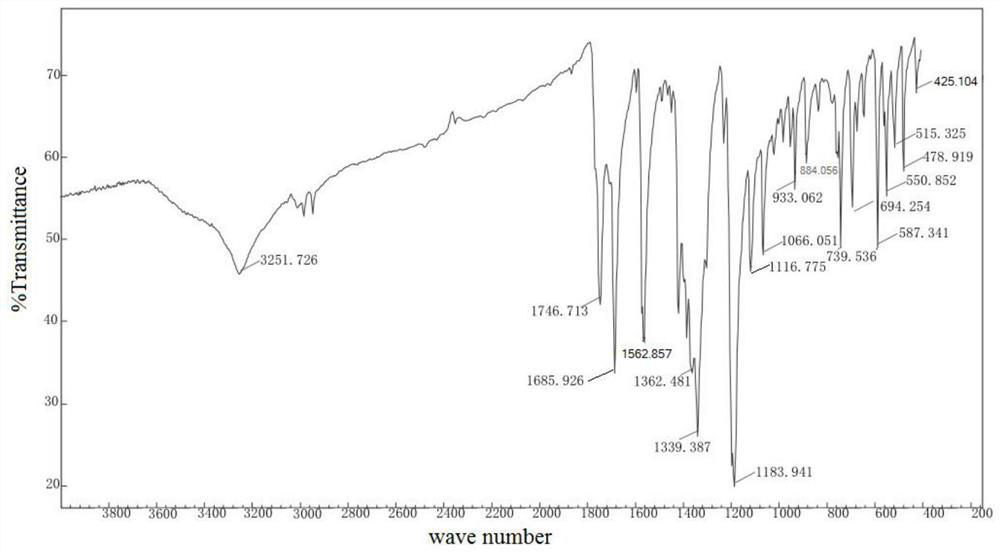
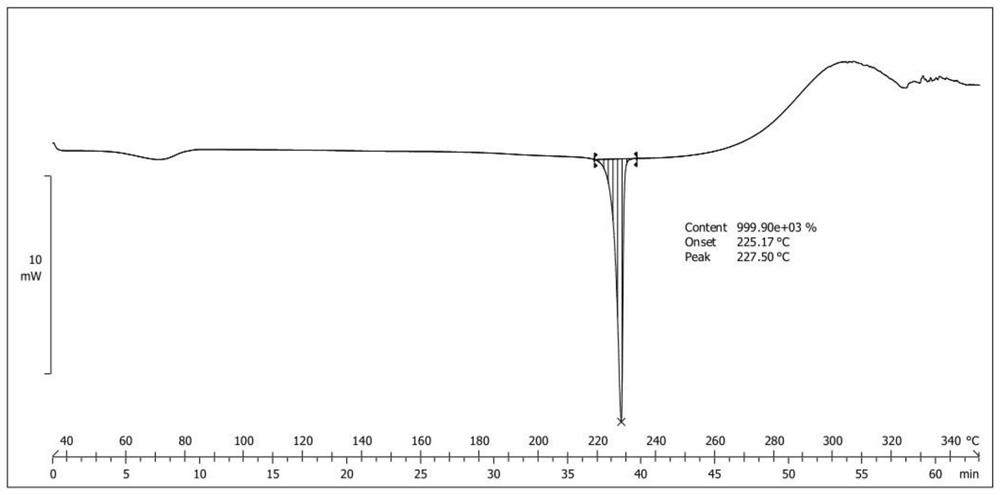
New epalrestat crystal form as well as preparation method and application thereof
A technology of epalrestat and crystal form, which is applied in the field of new crystal form of epalrestat and its preparation, can solve the problems of affecting drug bioavailability, affecting dissolution, poor water solubility of epalrestat, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a method for preparing the new crystal form of epalrestat described in the above technical scheme, comprising the following steps:
[0049] Carboxymethylrhodanine and α-methylcinnamaldehyde are condensed in a reaction system including a reaction solvent, an organic basic catalyst and a phase transfer catalyst to obtain a condensation reaction feed liquid;
[0050] Mixing the condensation reaction feed liquid and the acid liquid to obtain the crude product of epalrestat;
[0051] The crude epalrestat, alcohol solvent and acid solution are mixed and refined to obtain the new crystal form of epalrestat.
[0052] In the present invention, unless otherwise specified, the raw materials used in the present invention are preferably commercially available products.
[0053] In the invention, 3-carboxymethyl rhodanine and alpha-methyl cinnamaldehyde are condensed in a reaction system including a reaction solvent, an organic basic catalyst and ...
Embodiment 1
[0080] Add 320mL of purified water and 40080mL of polyethylene glycol in turn to a 1000mL three-neck round bottom flask, and add 3-carboxymethylrhodanine (15.3g, 80mmol) and α-methylcinnamaldehyde (14.0g, 96mmol) in turn under stirring at room temperature After the addition, 3-dimethylaminopropylamine (8.0 mL, 64 mmol) was added dropwise at a rate of 5 mL / min. After the dropwise addition, the temperature was raised to 60° C. and the stirring was continued (300 rpm) for 2.0 h. Turn off the heating and lower the temperature naturally, and at the same time add 20.0 mL of concentrated hydrochloric acid dropwise to the reaction system. After the reaction system was cooled to 25°C, it was filtered, the filter cake was rinsed with 100 mL of purified water, and dried under vacuum at 40°C for 2.0 h to obtain crude epalrestat (24.5 g, 96%) as a yellow solid.
[0081]In a 250mL round-bottomed flask, the crude product of epalrestat (16.0g, 50mmol) was suspended in 100mL of anhydrous meth...
Embodiment 2
[0104] Add 320mL of purified water and 40080mL of polyethylene glycol in turn to a 1000mL three-neck round bottom flask, and add 3-carboxymethylrhodanine (15.3g, 80mmol) and α-methylcinnamaldehyde (14.0g, 96mmol) in turn under stirring at room temperature After the addition, 3-dimethylaminopropylamine (8.0 mL, 64 mmol) was added dropwise at a rate of 5 mL / min. After the dropwise addition, the temperature was raised to 60° C. and the stirring was continued (300 rpm) for 2.0 h. Turn off the heating and lower the temperature naturally, and at the same time add 20.0 mL of concentrated hydrochloric acid dropwise to the reaction system. After the reaction system was cooled to 25°C, it was filtered, the filter cake was rinsed with 100 mL of purified water, and dried under vacuum at 40°C for 2.0 h to obtain crude epalrestat (24.5 g, 96%) as a yellow solid.
[0105] In a 250mL round bottom flask, the crude product of epalrestat (16.0g, 50mmol) was suspended in 120mL of isopropanol, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com