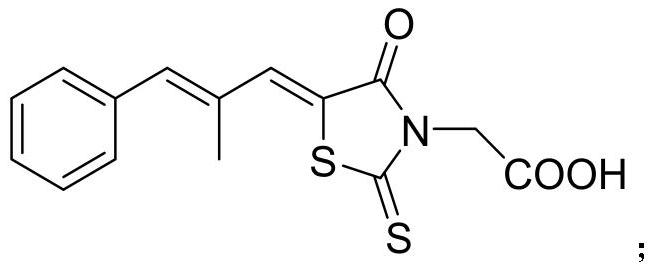
Preparation method of epalrestat
A technology of epalrestat and catalyst, which is applied in the field of preparation of epalrestat, and can solve problems such as strong corrosion, safety accidents, unsuitability for industrial scale-up production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of epalrestat, comprises the following steps:
[0025] 3-carboxymethylrhodanine, α-methylcinnamaldehyde, catalyst and water are mixed, followed by condensation reaction and acid neutralization to obtain crude epalrestat; the catalyst includes a basic catalyst and a phase transfer catalyst ;
[0026] The epalrestat crude product is mixed with an alcoholic organic solvent for recrystallization to obtain the epalrestat.
[0027] In the present invention, unless otherwise specified, all preparation materials are commercially available products well known to those skilled in the art.
[0028] In the present invention, 3-carboxymethylrhodanine, α-methylcinnamaldehyde, catalyst and water are mixed, followed by condensation reaction and acid neutralization to obtain the crude product of epalrestat; the catalyst includes a basic catalyst and a phase transfer catalyst.
[0029] In the present invention, the basic catalyst pre...
Embodiment 1
[0048] After mixing 3.2L of purified water and 0.8L of polyethylene glycol 400, 3-carboxymethylrhodanine (152.8g, 0.80mol) and α-methylcinnamaldehyde (140.0g, 0.96mol), then finally dropwise added 3-dimethylaminopropylamine (80.0mL, 0.64mol), kept stirring at 60°C for 2h, added 200mL concentrated hydrochloric acid (mass concentration was 37%) while cooling naturally, and cooled naturally After filtration, the obtained filter cake was rinsed with 1.0L of purified water, and dried in vacuum at 40°C for 2.0h to obtain crude epalrestat (yellow solid, 245.0g, 96%);
[0049] The epalrestat crude product (160.0g, 0.5mol) was mixed with 1000mL methanol, kept and stirred at 60°C for 2.0h, after natural cooling, filtered, and the obtained filter cake was rinsed with 200mL methanol, 40°C After vacuum drying for 2.0 h, epalrestat (139.2 g, yield 87%, purity 99.76%) was obtained.
[0050] The nuclear magnetic data of described epalrestat is: 1 H NMR (400MHz, CDCl 3 )δ13.46 (br, 1H), 7.6...
Embodiment 2
[0052] After mixing 3.6L of purified water and 0.4L of polyethylene glycol 400, 3-carboxymethylrhodanine (152.8g, 0.80mol) and α-methylcinnamaldehyde (140.0g, 0.96mol), then finally dropwise added 3-dimethylaminopropylamine (78.0mL, 0.64mol), kept stirring at 60°C for 2h, added 200mL concentrated hydrochloric acid (mass concentration was 37%) while naturally cooling, and cooled naturally After filtration, the obtained filter cake was rinsed with 1.0L of purified water, and dried in vacuum at 40°C for 2.0h to obtain crude epalrestat (yellow solid, 211.8g, 83%);
[0053] The epalrestat crude product (160.0g, 0.5mol) was mixed with 1000mL methanol, kept and stirred at 60°C for 2.0h, after natural cooling, filtered, and the obtained filter cake was rinsed with 200mL methanol, 40°C After vacuum drying for 2.0 h, epalrestat (136 g, yield 85%, purity 99.68%) was obtained.
[0054] The nuclear magnetic data of described epalrestat is: 1 H NMR (400MHz, CDCl 3 )δ13.46 (br, 1H), 7.63 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com