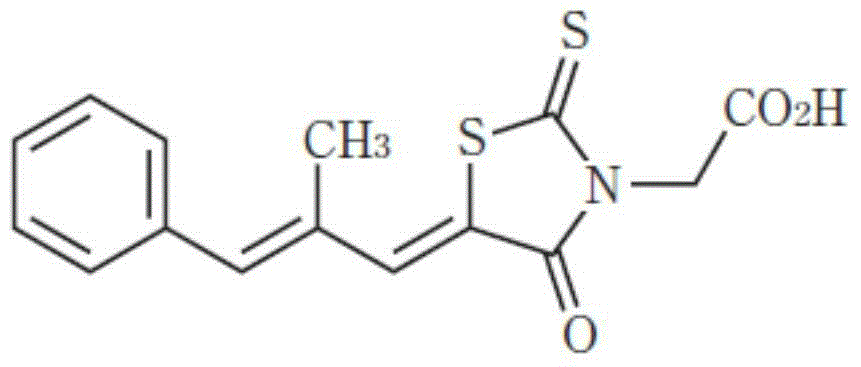
Epalrestat enteric-coated and sustained-release tablets and preparation method thereof
A technology of epalrestat and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., which can solve unfavorable industrial production, too fast alkali release, and uneven release and other problems, to achieve the effects of easy scale-up production, slow release, and excellent sustained-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
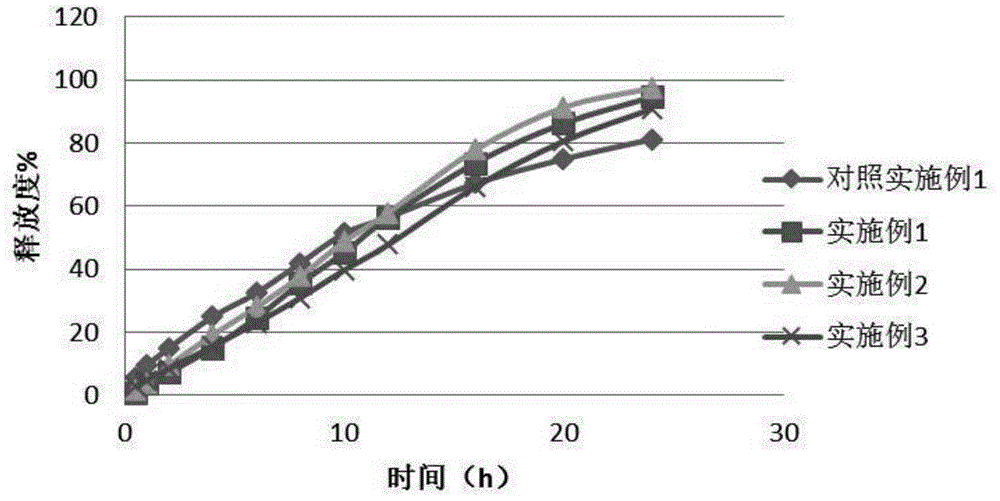
Image
Examples
Embodiment 1
[0039] Patent WO2012100208A1 provides a preparation of epalrestat sustained-release tablets (150mg / tablet, a total of 1000 tablets)
[0040] Inner granular material:
[0041]
[0042] Prelubrication:
[0043] Colloidal silica 2.5
[0044] Talc 2.5
[0045] lubricating:
[0046] Hydrogenated vegetable oil 6g
[0047] Preparation method: Weigh the prescription amount of API, mannitol, hypromellose and sodium alginate and put them into a three-dimensional motion mixer and mix them for 10 minutes; put the mixed powder into a wet granulator, and use purified water to make soft materials. Use swinging granules to granulate with a 16-mesh sieve; put the wet granules on a tray and dry in a blast drying oven at 50°C for 90 minutes; pass the dry granules through a 30-mesh sieve for granulation; put the dry granules into three-dimensional motion mixing The prescription amount of colloidal silicon dioxide and talcum powder is added to the machine for pre-lubrication; the prescript...
Embodiment 2
[0056] Preparation of epalrestat enteric-coated sustained-release tablets (150mg / tablet, 1000 tablets in total)
[0057] Single-layer core:
[0058]
[0059]
[0060] Slow-release coating:
[0061]
[0062] Preparation method: pulverize epalrestat, hydroxypropyl methylcellulose (added in K4M), lactose, and povidone (K30), pass through a 100-mesh sieve, mix mechanically, add an appropriate amount of purified water during stirring, and granulate , drying, whole grain. Then add hydroxypropyl methylcellulose (K4M extra) and magnesium stearate to the dry granules after the above-mentioned granulation, place in a granulator and mix evenly, control the hardness of the tablet at 14-16Kg, and press the tablet to obtain Single layer core. Disperse 7.5g of talcum powder in 63g of purified water and stir evenly, then add 1.5g of triethyl citrate and 0.5g of yellow iron oxide and continue to stir evenly. Add the above suspension to 50g For L 30D-55, coat after stirring evenl...
Embodiment 3
[0064] Preparation of epalrestat enteric-coated sustained-release tablets (150mg / tablet, 1000 tablets in total)
[0065] Single-layer core:
[0066]
[0067] Slow-release coating:
[0068]
[0069] Preparation method: pulverize epalrestat, hydroxypropyl methylcellulose (added in K4M), lactose, and povidone (K30), pass through a 100-mesh sieve, mix mechanically, and add 5% povidone during stirring (K30) Appropriate amount of aqueous solution, granulate, dry, and granulate. Then add hydroxypropyl methylcellulose (K4M extra) and magnesium stearate to the dry granules after the above-mentioned granulation, place in a granulator and mix evenly, control the hardness of the tablet at 14-16Kg, and press the tablet to obtain Single layer core. Disperse 7.5g of talcum powder in 63g of purified water and stir evenly, then add 1.5g of triethyl citrate and 0.5g of yellow iron oxide and continue to stir evenly. Add the above suspension to 50g For L 30D-55, coat after stirring ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com