Compound sustained-release tablet of epalrestat and sitagliptin or pharmaceutically acceptable salt thereof and preparation method thereof
A technology of compound sustained-release tablets and epalrestat, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
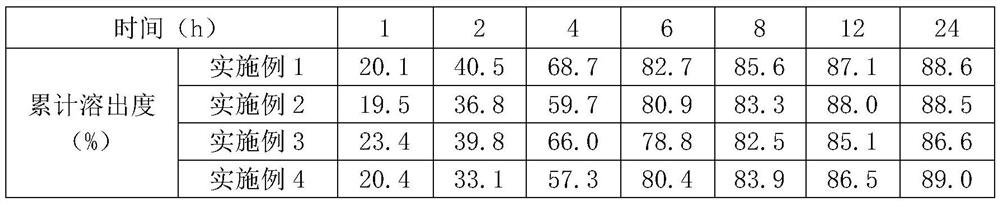
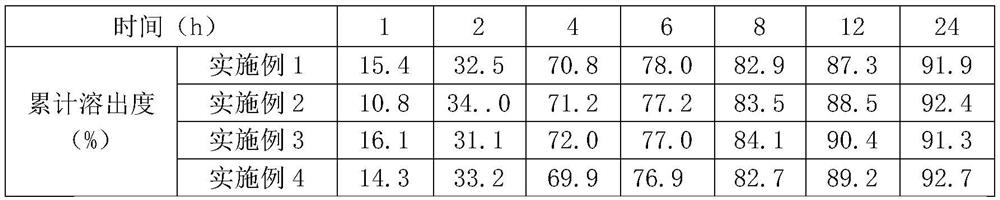
Embodiment 1
[0040] Embodiment 1: Epalrestat, sitagliptin film-controlled matrix sustained-release single-layer tablet (1000 tablets)
[0041] (1) Chip part
[0042] Epalrestat 75g, sitagliptin phosphate 50g, calcium hydrogen phosphate anhydrous 45g, mannitol 80g, hydroxypropyl methylcellulose 60g, hydroxypropyl cellulose 6g, croscarmellose sodium 5g, Colloidal silicon dioxide 2g, sodium stearyl fumarate 5g, magnesium stearate 2g;
[0043] (2) Membrane-controlled slow-release coating part
[0044] Ethyl cellulose 18g, hypromellose 5g, diethyl phthalate 2g, titanium dioxide 5g
[0045] Preparation method:
[0046](1) Pretreatment of raw materials and auxiliary materials: Pass epalrestat, sitagliptin or their pharmaceutically acceptable salt raw materials through a 100-mesh sieve, and pass fillers and other auxiliary materials through a 60-mesh sieve.
[0047] (2) Wet granulation: Weigh raw materials and mix them evenly with auxiliary materials other than hydroxypropyl cellulose, glidant...
Embodiment 2
[0051] Embodiment 2: Epalrestat, sitagliptin film-controlled matrix sustained-release single-layer tablet (1000 tablets)
[0052] (1) Chip part
[0053] Epalrestat 75g, sitagliptin 50g, calcium hydrogen phosphate anhydrous 50g, microcrystalline cellulose 52g, mannitol 35g, carboxymethyl cellulose 60g, hydroxypropyl methyl cellulose 5g, croscarmellose Sodium cellulose 6g, colloidal silicon dioxide 2g, sodium stearyl fumarate 4g, magnesium stearate 2g;
[0054] (2) Membrane controlled release coating material
[0055] Cellulose acetate 14g, hypromellose 9g, polyoxyethylene polyoxypropylene glycol 2g, titanium dioxide 5g.
[0056] Preparation method: consistent with the preparation method in Example 1, the core part of the tablet is changed from the hydroxypropyl cellulose aqueous solution in Example 1 as binder granulation to mixed granulation with purified water as wetting agent.
Embodiment 3
[0057] Embodiment 3: Epalrestat, sitagliptin film-controlled matrix sustained-release double-layer tablet (1000 tablets)
[0058] (1) Chip part
[0059] Epalrestat sustained-release layer: 75g of epalrestat, 42g of mannitol, 12g of lactose, 35g of hydroxypropyl methylcellulose, 4g of carboxymethylcellulose calcium, 2g of magnesium stearate;
[0060] Sitagliptin or its pharmaceutically acceptable salt immediate-release layer: 50g of sitagliptin phosphate, 50g of anhydrous calcium hydrogen phosphate, 40g of mannitol, 3g of croscarmellose sodium, 5g of sodium stearyl fumarate, Magnesium stearate 2g;
[0061] (2) Membrane-controlled slow-release coating part
[0062] Eudragit RS 14g, Eudragit RL 4g, polyethylene glycol 4g, diethyl phthalate 3g, titanium dioxide 5g.
[0063] Preparation method:
[0064] (1) Pretreatment of raw materials and auxiliary materials: Pass epalrestat, sitagliptin or their pharmaceutically acceptable salt raw materials through a 100-mesh sieve, and pas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com