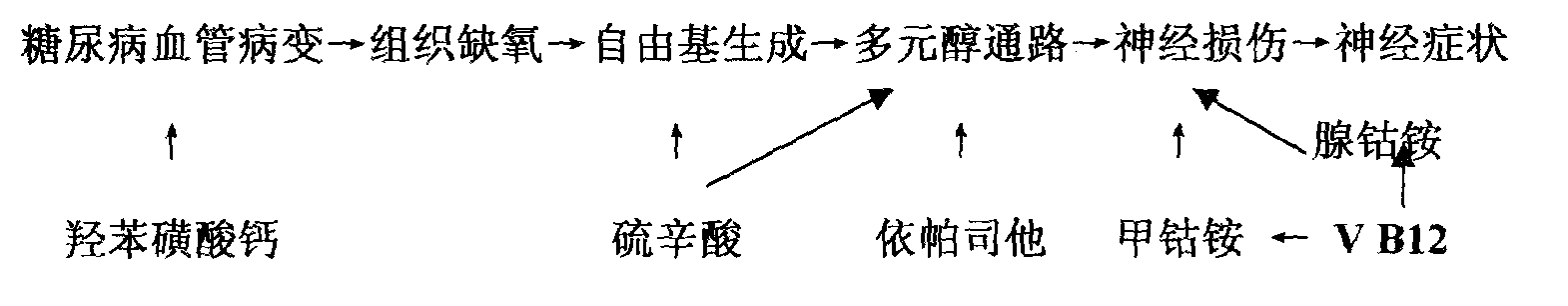
Medicinal composition for treating diabetes chronic complication and preparation method thereof
A composition and diabetes technology, applied in the field of medicine, can solve problems such as literature and patent reports on compound preparations that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 lipoic acid and methylcobalamin tablet
[0024] prescription:
[0025] components
Dosage
lipoic acid
Mecobalamin
CMS-Na
Micropowder silica gel
Co-made
100g
0.5g
90g
5g
4.5g
1000 pieces
[0026] Preparation:
[0027] Pass lipoic acid and microcrystalline cellulose through a 80-mesh sieve, mix well, and set aside; take another appropriate amount of 50% ethanol solution, add methylcobalamin to dissolve, use this solution as a binder to make a soft material, and granulate with a 24-mesh sieve , dried at 50°C, granulated with a 20-mesh sieve, added with micropowdered silica gel and CMS-Na, mixed evenly, and pressed into tablets with a suitable die to obtain the product.
[0028] If the above-mentioned tablet is coated, a coated tablet can be obtained, which can be a film-coated tablet, an enteric-coated tablet, and the like....
Embodiment 2
[0030] Embodiment 2: compound methylcobalamin+lipoic acid+calcium dobesilate capsules
[0031] prescription:
[0032] components
Dosage
lipoic acid
Mecobalamin
Co-made
100g
0.5g
500g
10g
10g
1000 capsules
[0033] Preparation:
[0034] Pass lipoic acid and calcium dobesilate through an 80-mesh sieve, mix them evenly with starch, and set aside; take an appropriate amount of 50% ethanol solution, add methylcobalamin to dissolve, and use this solution as an adhesive to make a soft material. Granulate with a mesh sieve, dry at 50°C, granulate with a 20-mesh sieve, add magnesium stearate, mix evenly, pack separately, and obtain.
[0035] The used capsule shell can be a common capsule or an enteric-coated capsule.
[0036] The above steps should be strictly protected from light.
Embodiment 3
[0037] Example 3: Compound lipoic acid + epalrestat + calcium dobesilate and methylcobalamin chewable tablet
[0038] prescription:
[0039] components
Dosage
lipoic acid
calcium dobesilate
Micropowder silica gel
Co-made
100g
50g
500g
500g
30g
20g
1000 pieces
[0040] Preparation:
[0041] Pass lipoic acid, epalrestat, and calcium dobesilate through an 80-mesh sieve, mix them evenly with sorbitol, take an appropriate amount of 50% ethanol solution, use it as a soft material for binder, granulate with a 24-mesh sieve, and heat at 50°C Dry and sieve through a 20-mesh sieve. Add sweet orange powder essence and micro-powder silica gel, mix evenly, and press into tablets to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com