Novel choline cocrystal of epalrestat
A technology of co-crystallization and choline dihydrogen, which is applied in the direction of medical preparations containing active ingredients, metabolic diseases, extracellular fluid diseases, etc., can solve the problem that there is no way to predict the co-crystal characteristics of compounds, and it takes a lot of time, issues of effort and resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1-intermediate product
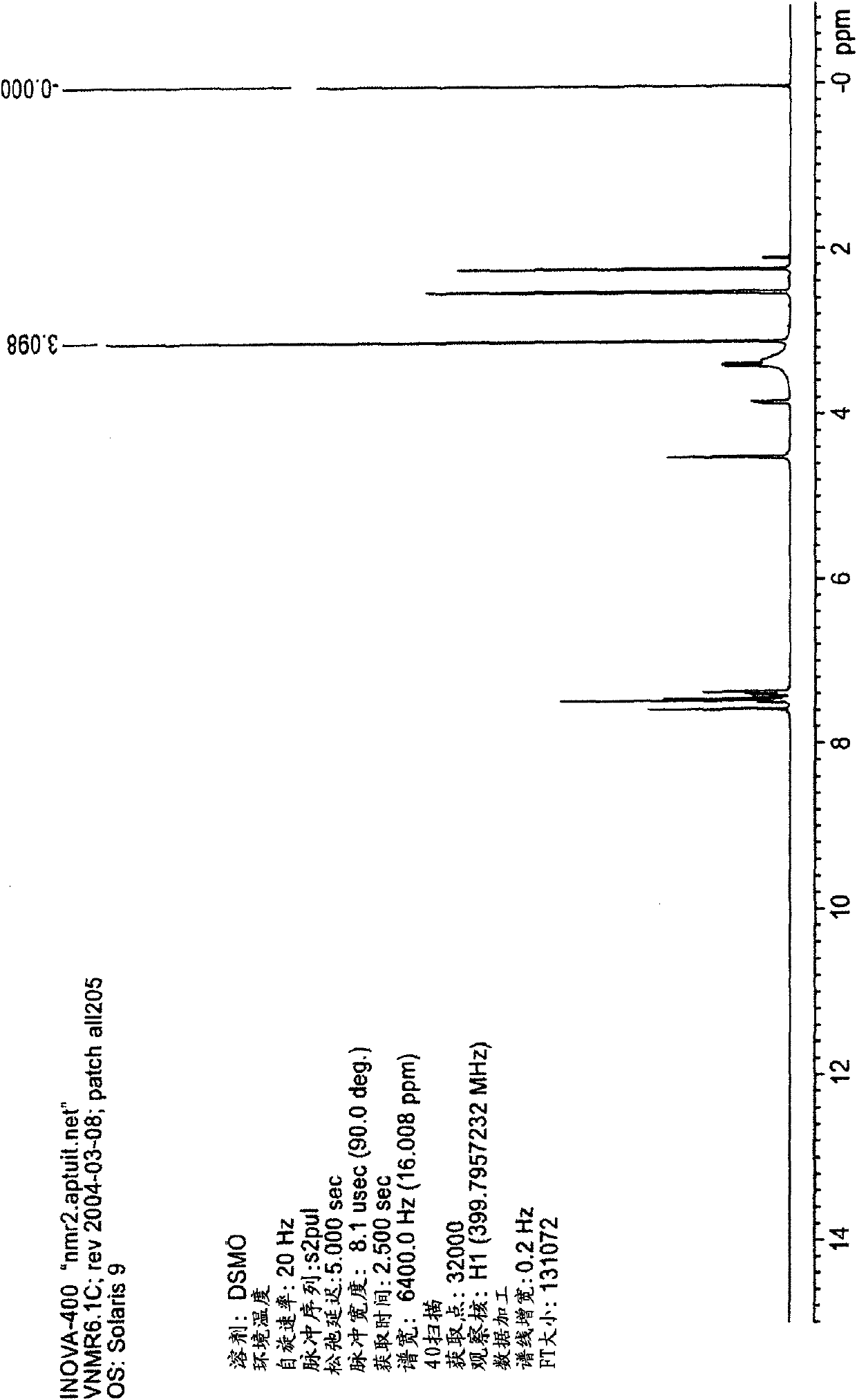
[0064] With 0.187mL 50% choline hydroxide aqueous solution (0.187mL, with a density of 1.073g / mL = 201mg 50% choline hydroxide (by weight) = 101mg, 0.83mmol choline hydroxide) treatment 265mg (0.83mmol) A slurry of parerestat free acid and 20 mL of absolute ethanol. During the addition, all solids dissolved. The resulting solution was poured into 400 mL of diethyl ether and the precipitated solid was collected by filtration to give 282 mg of product. The solid was dried overnight in a vacuum oven at 1-5 Torr pressure at ambient temperature. Collect solid, obtain analysis data to intermediate product: XRPD pattern such as Figure 4 as shown, 1 H-NMR spectrum as Figures 5A-5C shown.
Embodiment 2
[0065] Preparation of the choline diacid hydrogen cocrystal of embodiment 2-epalrestat
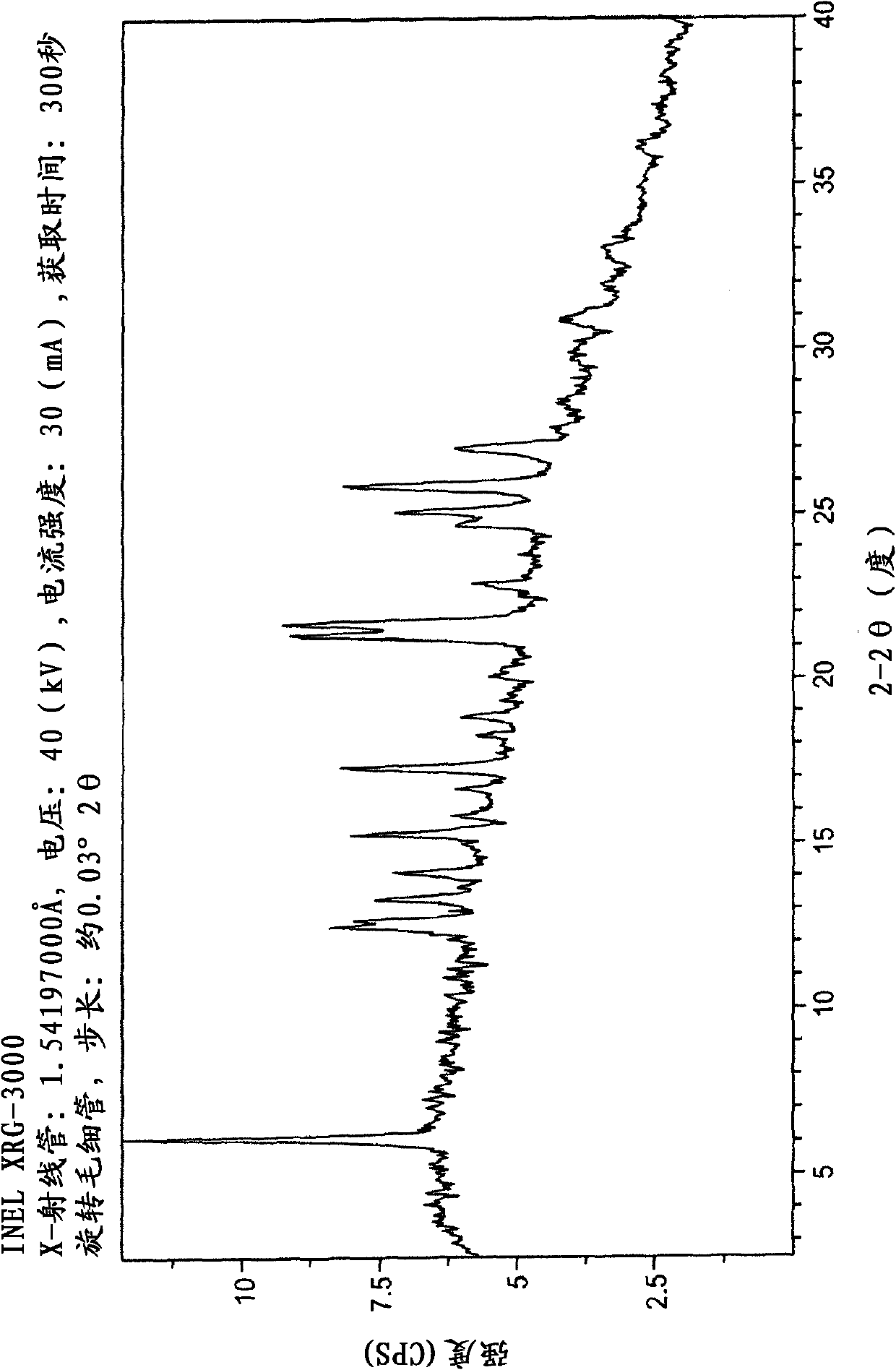
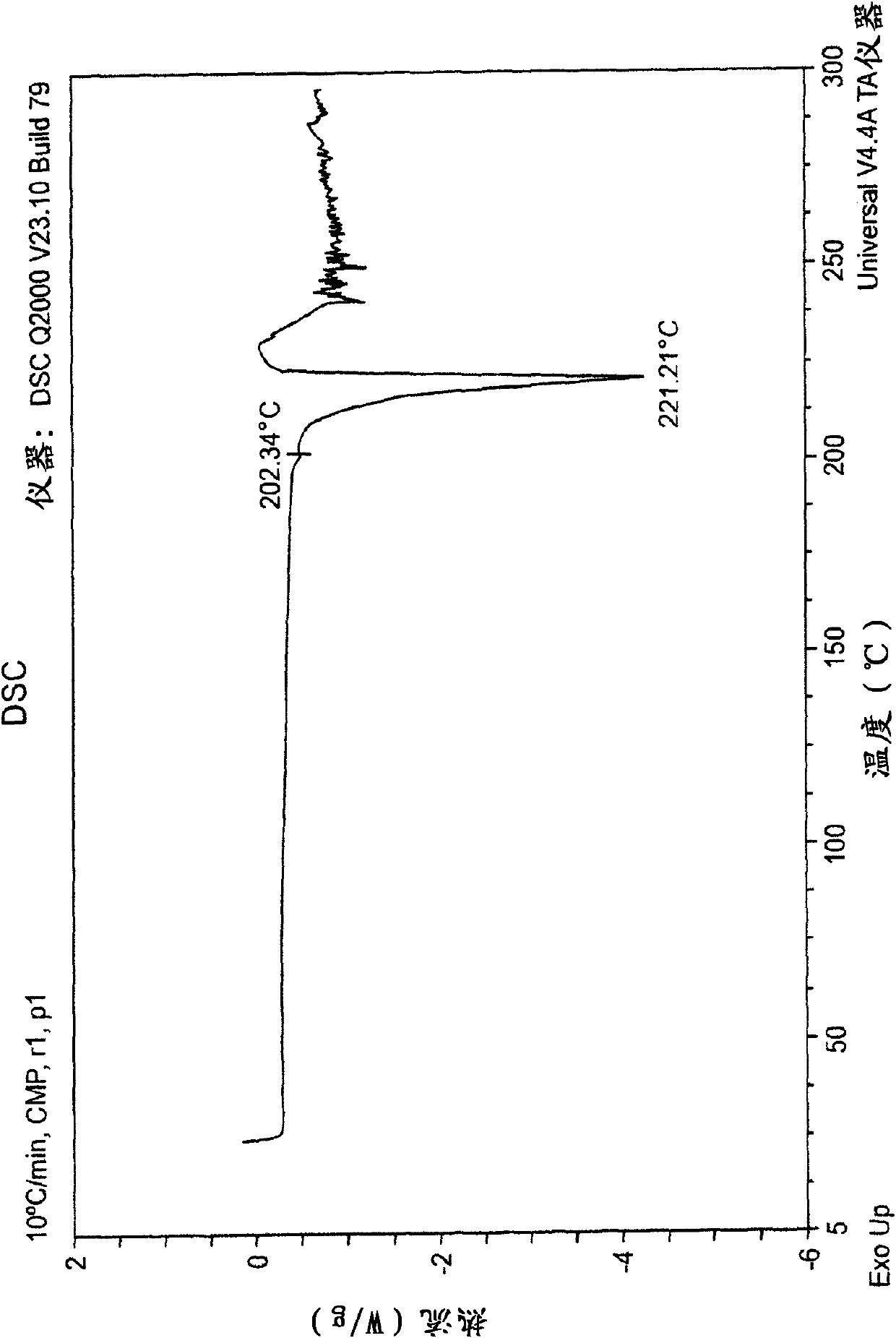
[0066] Briefly sonicate a mixture of 209 mg epalrestat free acid and 11 mL acetone and filter through a 0.2 micron nylon filter. The filtrate was treated with 39 mg of the intermediate product of Example 1 to give a slurry. The slurry was stirred for about 4 days by placing it in a capped vial on a rotating wheel. Filtration gave 56 mg of di-epalrestat hydrocholine as a solid. Obtain analytical data on the product: XRPD patterns such as figure 1 As shown, the DSC thermal analysis chart is shown as figure 2 as shown, 1 H-NMR spectrum as Figure 3A-3D shown. Elemental analysis is as follows:
[0067]
Embodiment 3
[0068] Preparation of the choline diacid hydrogen cocrystal of embodiment 3-epalrestat
[0069] Briefly sonicate a mixture of 85 mg of epalrestat free acid and 20 mL of absolute ethanol and filter through a 0.2 micron nylon filter. The filtrate was treated with 26 mg of the intermediate product of Example 1 while sonicating to give a slurry. The slurry was stirred for about 4 days by placing it in a capped vial on a rotating wheel. Filtration gave 36 mg of di-epalrestat hydrocholine as a solid. Obtain analytical data to the product: the XRPD pattern is basically as figure 1 shown. Solubility data in water were obtained for the final product, with solubility properties such as Figure 6A shown. as can be found in Figure 6A As observed in , the dissolved concentration of epalrestat increased over time to 249 μg / mL after about 24 hours. as can be found in Figure 6B As observed in , the choline dihydrogen diacid cocrystal of epalrestat shows a solubility in water that is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com