Kurarinone osmotic pump controlled release tablets and preparation method thereof
An osmotic pump controlled release and matrine technology, which is applied in osmotic transportation, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of failure to achieve stable and constant release of drugs, difficulty in large-scale production, and differences in pore size Larger problems, to achieve the effect of inhibiting collagen activity, avoiding peak and valley phenomena, and preventing liver fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
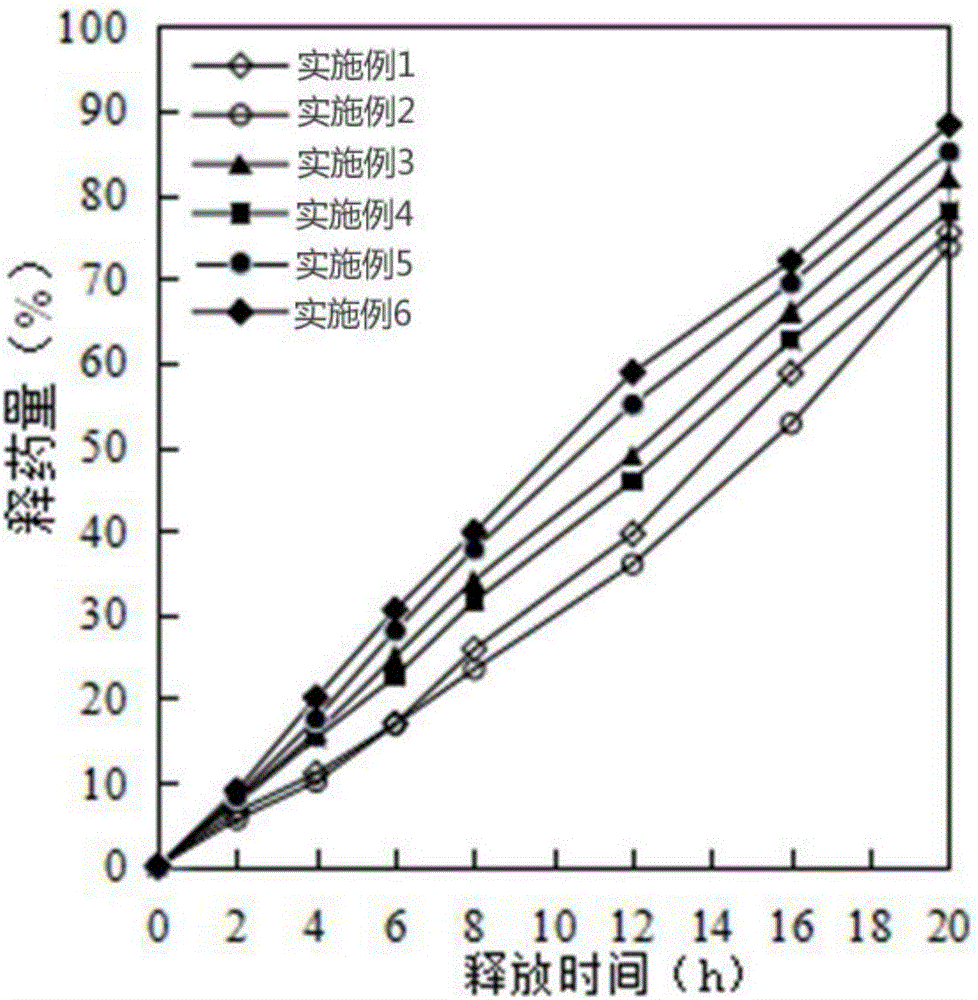
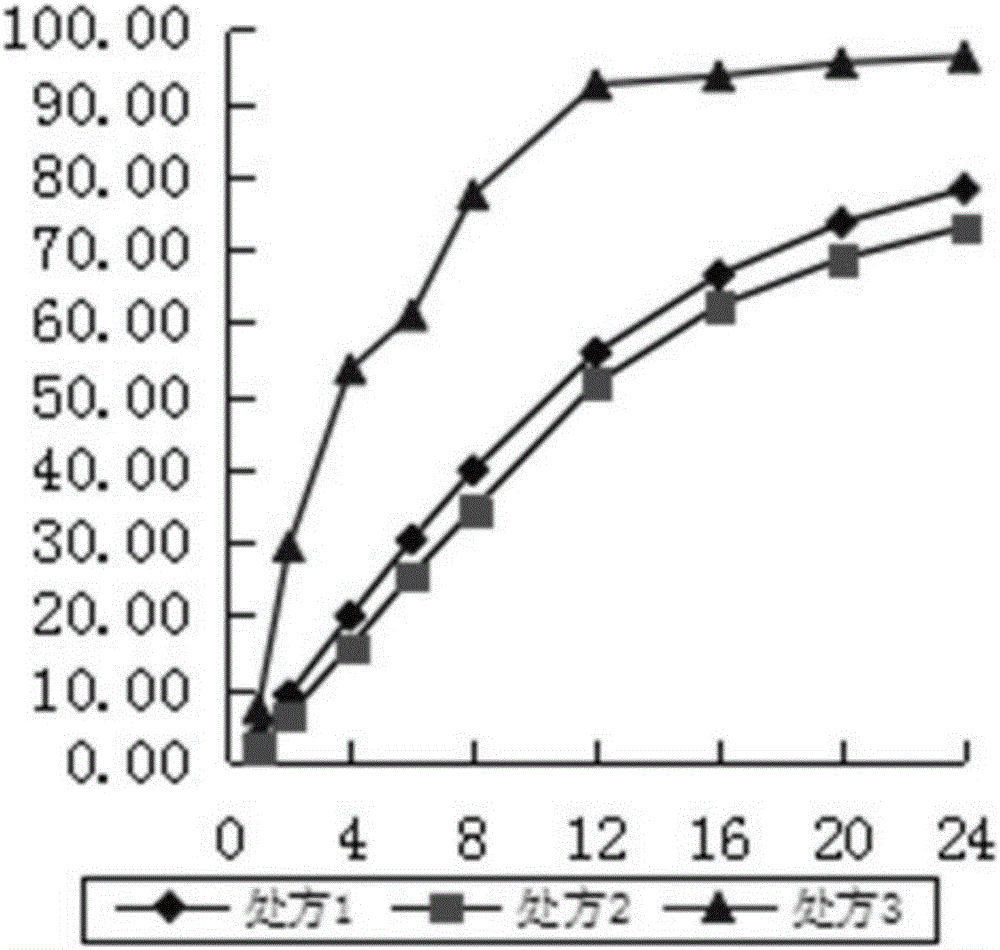
Examples
Embodiment 1
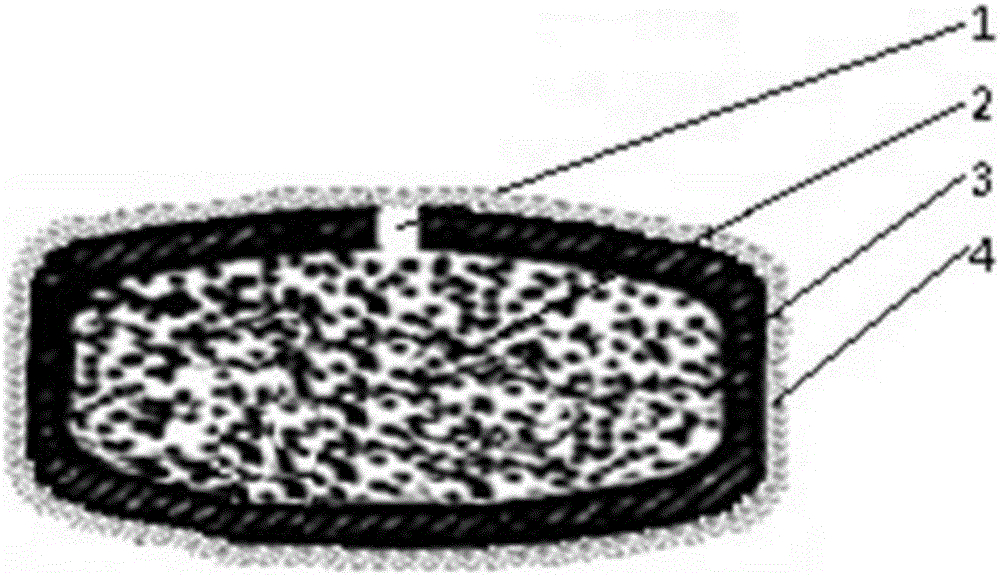
[0066] See figure 1 As shown, a matrine osmotic pump controlled-release tablet is composed of a plain tablet and a moisture-proof protective film 4 wrapped outside the plain tablet. The plain tablet is composed of a tablet core 2 and a semipermeable membrane 3 wrapped outside the tablet core 2. A drug release hole 1 is provided on the semipermeable membrane 3; the drug release hole 1 is formed by laser drilling, and the size of the hole is 0.5mm.
[0067] Tablet core 2 is made up of the raw material of following weight percentage content:
[0068] 40.00% matrine, 21.00% sodium chloride, 30.00% lactose, 5.00% hydroxypropyl methylcellulose, 3.50% polyvinylpyrrolidone, 0.50% magnesium stearate; the weight of the tablet core is 450.00 mg.
[0069] The semipermeable membrane coating liquid that prepares semipermeable membrane 3 is made up of the raw material of following weight percent content:
[0070] Cellulose acetate 3.50%, polyethylene glycol-40000.25%, diethyl phthalate 0.3...
Embodiment 2
[0083] See figure 1 As shown, a matrine osmotic pump controlled-release tablet is composed of a plain tablet and a moisture-proof protective film 4 wrapped outside the plain tablet. The plain tablet is composed of a tablet core 2 and a semipermeable membrane 3 wrapped outside the tablet core 2. A drug release hole 1 is provided on the semipermeable membrane 3; the drug release hole 1 is formed by laser drilling, and the size of the hole is 0.5mm.
[0084] Tablet core 2 is made up of the raw material of following weight percentage content:
[0085] 40.00% matrine, 25.00% sodium chloride, 26.00% lactose, 4.75% hydroxypropyl methylcellulose, 2.75% polyvinylpyrrolidone, 1.50% magnesium stearate; the weight of the tablet core is 450.00mg.
[0086] The semipermeable membrane coating liquid that prepares semipermeable membrane 3 is made up of the raw material of following weight percent content:
[0087] Cellulose acetate 3.50%, polyethylene glycol-40000.25%, diethyl phthalate 0.35...
Embodiment 3
[0091] See figure 1 As shown, a matrine osmotic pump controlled-release tablet is composed of a plain tablet and a moisture-proof protective film 4 wrapped outside the plain tablet. The plain tablet is composed of a tablet core 2 and a semipermeable membrane 3 wrapped outside the tablet core 2. A drug release hole 1 is provided on the semipermeable membrane 3; the drug release hole 1 is formed by laser drilling, and the size of the hole is 0.5mm.
[0092] Tablet core 2 is made up of the raw material of following weight percentage content:
[0093] 60.00% matrine, 15.00% sodium chloride, 20.00% lactose, 2.75% hydroxypropyl methylcellulose, 1.25% polyvinylpyrrolidone, 1.00% magnesium stearate; the weight of the tablet core is 350.00mg.
[0094] The semipermeable membrane coating liquid that prepares semipermeable membrane 3 is made up of the raw material of following weight percent content:
[0095] Cellulose acetate 3.80%, polyethylene glycol-40000.27%, diethyl phthalate 0.38...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com