Compound control-released percutaneous medicine plaster for treating high blood pressure and its preparation method
A high blood pressure and drug technology, which is applied in the field of compound transdermal controlled-release patches for the treatment of high blood pressure drugs and their preparation, can solve the problem of large individual differences in drug absorption and in vivo metabolism, inaccurate curative effects, and increased incidence of adverse reactions, etc. It can avoid the peak and valley phenomenon of blood drug concentration, improve the treatment efficiency and safety, and facilitate the individualized administration of dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The steps of the embodiment of the preparation method of the present invention include:
[0081] (1) Weigh the compound drug and compound transdermal penetration enhancer in proportion, dissolve them in absolute ethanol, stir at 1500-3000rpm for 20min, and form a clear solution, slowly add dibutyl sebacate dropwise in the above solution as a plasticizer agent, succinic acid as the cross-linking agent, stirring at 1500-3000rpm for 30min to make a clear solution;
[0082] (2) Add absolute ethanol to the required composite polymer material, swell naturally for 6 hours to form a transparent colloidal solution, combine the solutions of (1) and (2), and stir at 1500-3000rpm for 30min to form a clear solution containing Drug pressure sensitive adhesive reservoir viscose;
[0083] (3) apply the drug-containing pressure-sensitive adhesive reservoir viscose on the backing film with a coating machine to form a film with uniform thickness;
[0084] (4) Put the film into a blast d...
Embodiment 1
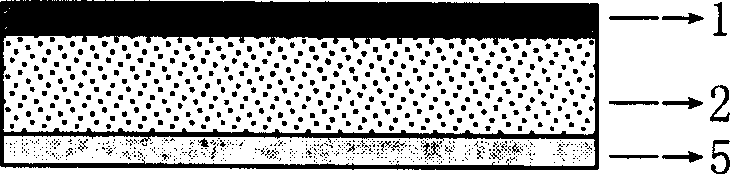
[0098] Embodiment 1: its composition structure is as figure 1 As shown, wherein: the backing film 1 adopts aluminum foil composite polyethylene film, and its thickness is about 0.05mm; 10; the mass ratio w / w accounting for the composite polymer material is 25%) and the composite transdermal penetration enhancer azone, propylene glycol, oleic acid and terpenoid turpentine (accounting for the mass ratio w / w of the composite polymer material is 25%) 3%) and the mixture of E, RS type polyacrylic resin and ethylene / vinyl acetate copolymer (EVA), its thickness is 1.6mm, protective film 5 adopts aluminum foil composite polyethylene film, and its thickness is about 0.05mm.
[0099] If it is necessary to better control the drug release rate and quickly reach the required blood drug concentration after the first administration, a rate-controlled release film and an immediate-release drug-containing pressure-sensitive adhesive layer can be sequentially added to the pressure-sensitive adh...
Embodiment 2
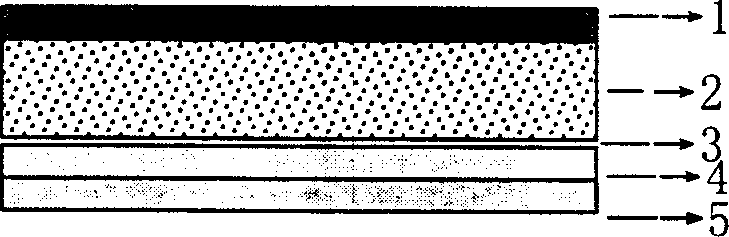
[0100] Embodiment 2: its composition structure is as figure 1 As shown, wherein: the backing film 1 adopts cellulose acetate film, and its thickness is about 0.1mm; Accounting for the mass ratio w / w of the composite polymer material is 15%) and composite transdermal penetration enhancer azone, propylene glycol, monolauric acid and terpenoid lemon essential oil (accounting for the mass ratio w / w of the composite polymer material is 5%) and the mixture of polyisobutylene and polyvinyl alcohol (PVA), its thickness is 1.4mm, can add protective film 5 now.
[0101] If it is necessary to better control the drug release rate and quickly reach the required blood drug concentration after the first administration, the rate-controlled release film and the immediate-release drug-containing pressure-sensitive adhesive layer can be sequentially added to the pressure-sensitive adhesive skeleton-type drug-containing reservoir layer, Such as figure 2 As shown, wherein: the rate-controlled r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com