Beta-cyclodextrin based pH responsive star polymer, micelle and composite material
A star-shaped polymer, cyclodextrin technology, applied in the directions of non-active components of polymer compounds, drug combinations, medical preparations of non-active components, etc., can solve the problems of dissociation and agglomeration of gold nanoparticles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
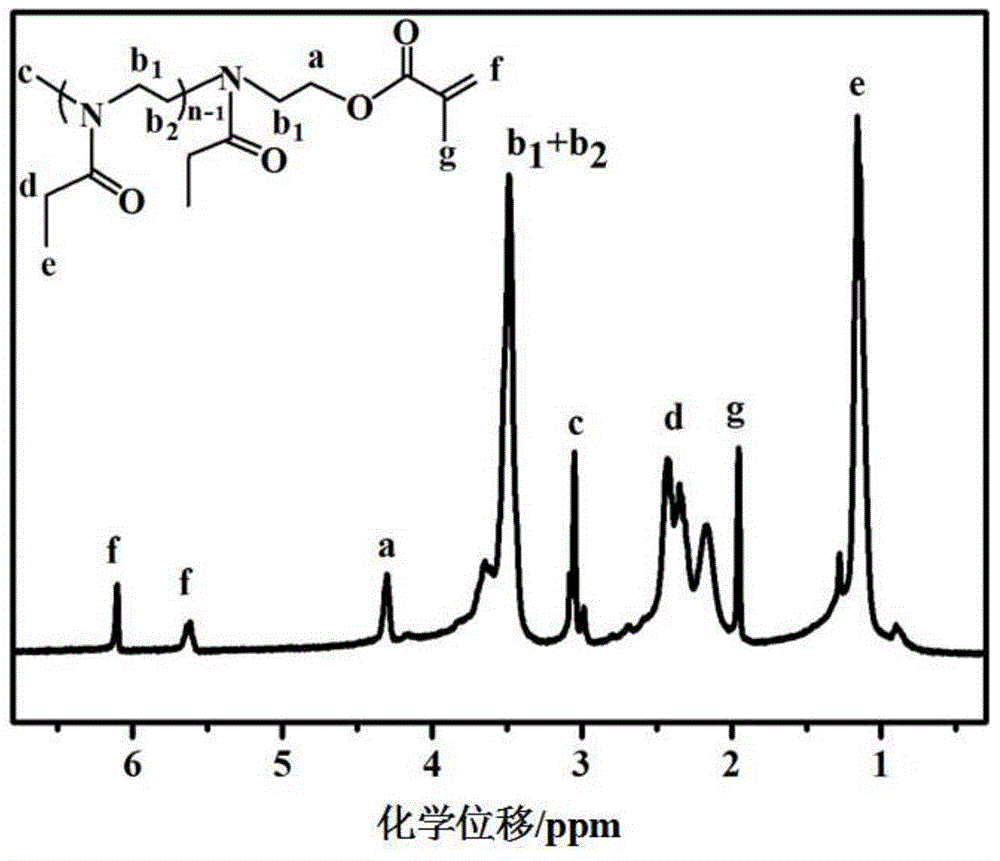
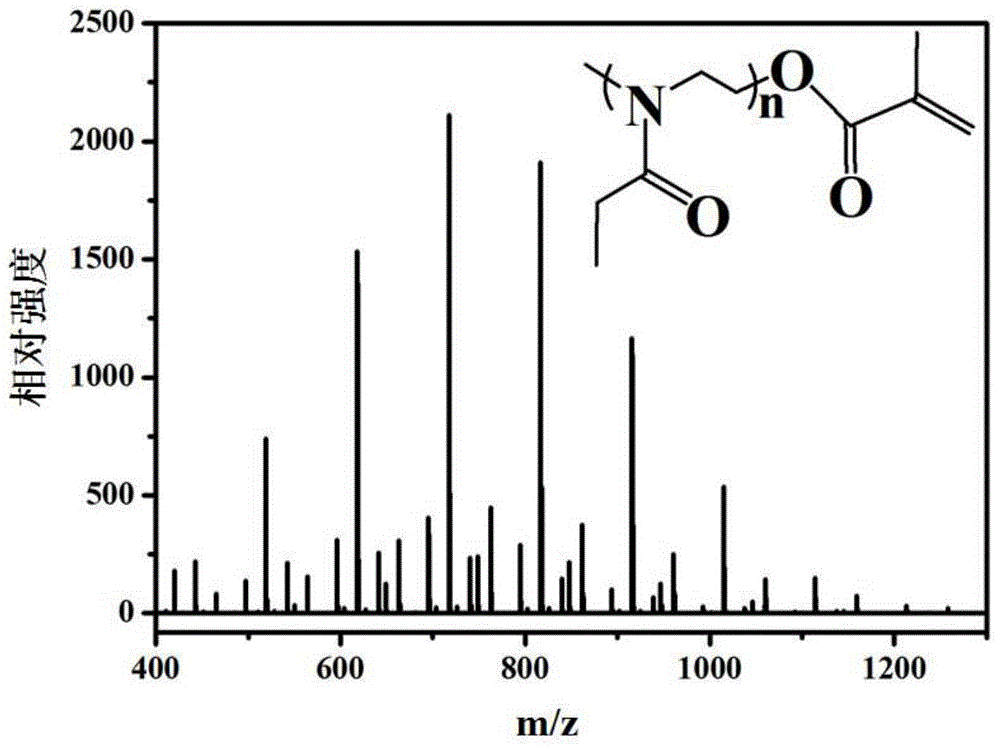
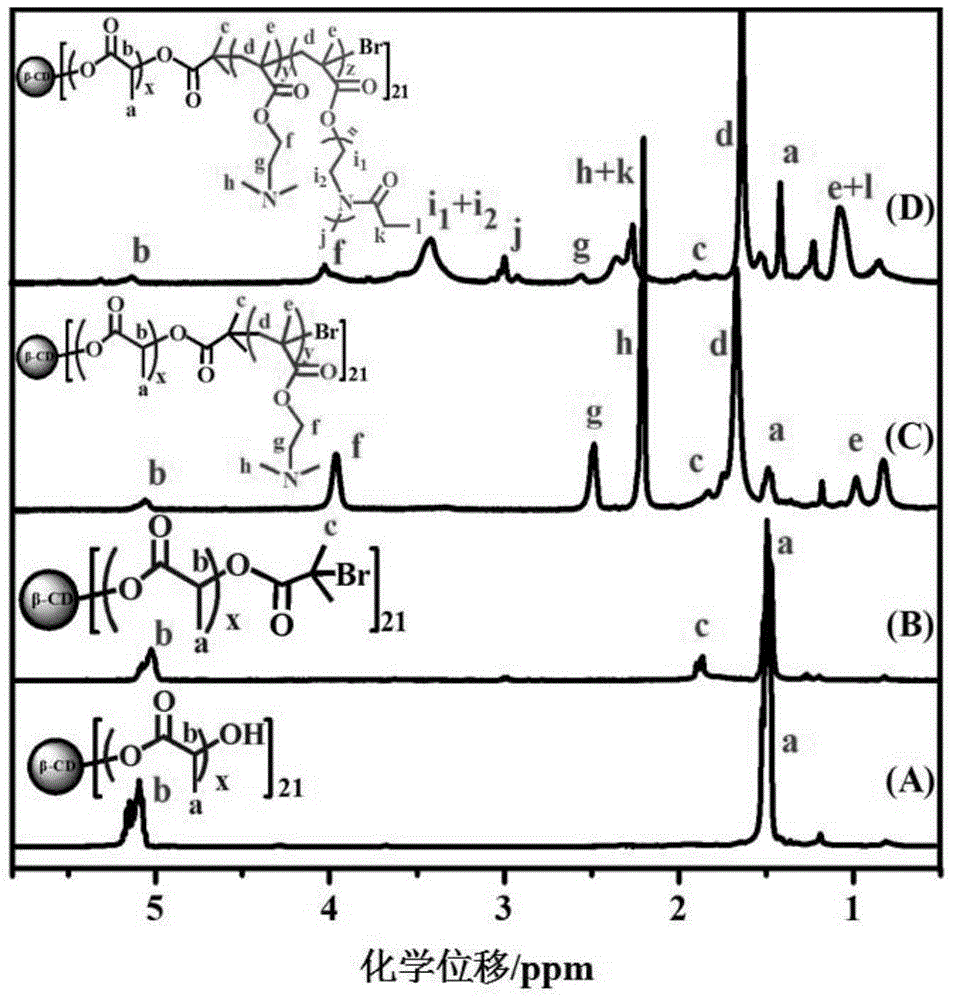
[0102] Embodiment 1: the preparation of hydrophilic macromer EtOxMA (n=7)
[0103] Under argon protection, methyl p-toluenesulfonate (MeTos, 2.49 g, 13.4 mmol), 2-ethyl-2-oxazoline (EtOx, 9.3 g, 94 mmol) and acetonitrile (6 mL) were mixed, After stirring for 30s, microwave radiation heated to 140°C, reacted for 3min and cooled to room temperature, then added methacrylic acid (MAA, 1.722g, 20mmol) and TEA (3.7mL) successively, and reacted in an oil bath at 80°C under the protection of argon 15h. After the reaction was completed, the acetonitrile was removed by rotary evaporation, and then the polymer was dissolved with chloroform (50 mL), and successively dissolved with NaHCO 3 solution (0.5M, 200mL) and NaCl solution (0.5M, 200mL), the organic phase was extracted with anhydrous MgSO 4 After drying and filtering, the solvent in the filtrate was removed by rotary evaporation, and vacuum-dried at 45 °C and 35 mb to obtain a white sticky solid, which was stored in argon at 20 °C...
Embodiment 2
[0105] Embodiment 2: the preparation of hydrophilic macromer EtOxMA (n=2)
[0106] Under argon protection, methyl p-toluenesulfonate (MeTos, 2.49 g, 13.4 mmol), 2-ethyl-2-oxazoline (EtOx, 2.67 g, 27 mmol) and acetonitrile (5 mL) were mixed, After stirring for 30s, microwave radiation heated to 140°C, reacted for 3min and cooled to room temperature, then added methacrylic acid (MAA, 1.722g, 20mmol) and TEA (3.7mL) successively, and reacted in an oil bath at 80°C under the protection of argon 15h. After the reaction was completed, the acetonitrile was removed by rotary evaporation, and then the polymer was dissolved with chloroform (50 mL), and successively dissolved with NaHCO 3 solution (0.5M, 200mL) and NaCl solution (0.5M, 200mL), the organic phase was extracted with anhydrous MgSO 4 After drying and filtering, the solvent in the filtrate was removed by rotary evaporation, and vacuum-dried at 45 °C and 35 mb to obtain a white sticky solid, which was stored in argon at 20 °...
Embodiment 3
[0107] Embodiment 3: the preparation of hydrophilic macromer EtOxMA (n=10)
[0108] Under argon protection, methyl p-toluenesulfonate (MeTos, 2.49 g, 13.4 mmol), 2-ethyl-2-oxazoline (EtOx, 13.35 g, 135 mmol) and acetonitrile (8 mL) were mixed, After stirring for 30s, microwave radiation heated to 140°C, reacted for 3min and cooled to room temperature, then added methacrylic acid (MAA, 1.722g, 20mmol) and TEA (3.7mL) successively, and reacted in an oil bath at 80°C under the protection of argon 15h. After the reaction was completed, the acetonitrile was removed by rotary evaporation, and then the polymer was dissolved with chloroform (50 mL), and successively dissolved with NaHCO 3 solution (0.5M, 200mL) and NaCl solution (0.5M, 200mL), the organic phase was extracted with anhydrous MgSO 4 After drying and filtering, the solvent in the filtrate was removed by rotary evaporation, and vacuum-dried at 45 °C and 35 mb to obtain a white sticky solid, which was stored in argon at 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Mn | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com