Nifedipine controlled-release tablet composition and preparation method thereof
A technology for nifedipine and composition, applied in the field of nifedipine controlled-release tablet composition and preparation thereof, can solve the problems of complicated preparation process, long drying time, slow water absorption speed and hydration speed of polyoxyethylene, and avoid the Pigment and toxic solvent, simple and easy preparation process, good release curve effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method of a nifedipine controlled-release tablet composition, comprising the following steps:
[0037] Step 1, pretreatment: prepare the raw material nifedipine and various auxiliary materials, and micronize the raw material nifedipine under the condition of avoiding light, so that the fineness can pass through a 200-mesh sieve, and the auxiliary materials pass through a 80-100 mesh sieve;
[0038] Step 2, granulation: under dark conditions, dissolve the adhesive in a wetting agent to obtain an adhesive solution, add the raw material nifedipine to the adhesive solution and stir evenly for later use; weigh polyoxyethylene, high The viscous hydrophilic skeleton material, the hydrophobic retarder and the filler are mixed evenly, and then added to the adhesive solution prepared in step 1 to make a soft material; granulation, drying, sizing, adding lubricant and mixing to obtain the granules;
[0039] Step 3, tabletting: under the condition of avoiding light, u...
Embodiment 1
[0042] Granulation: the raw material is micronized and passed through a 200-mesh sieve, the auxiliary material lactose is passed through a 100-mesh sieve, and other auxiliary materials are passed through a 80-mesh sieve; the binder povidone K30 is dissolved in ethanol and the raw material is stirred evenly for later use; the prescription amount of polyoxyethylene is weighed , hypromellose, ethyl cellulose, lactose and or sodium chloride are mixed evenly and added to the prepared binder solution to make a soft material, granulated with a 18-mesh sieve, dried in a hot air circulation oven at 50-60°C, and 18-mesh sieve The whole grain is blended with lubricant magnesium stearate.
[0043] Tablet compression: This step is carried out in the dark, and the granules prepared above are punched with a shallow arc with a diameter of 8.0mm using an ordinary tablet press machine, and the hardness is controlled at 4.0kg / cm 2 up to 12 kg / cm 2 The inspection is qualified and ready for use. ...
Embodiment 2-11
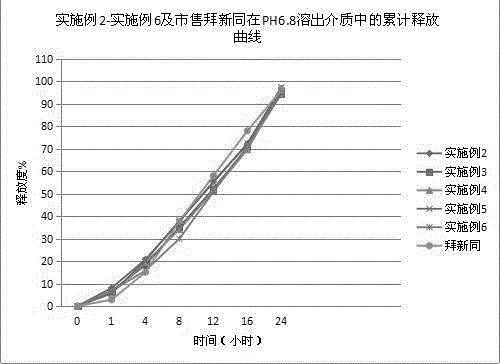
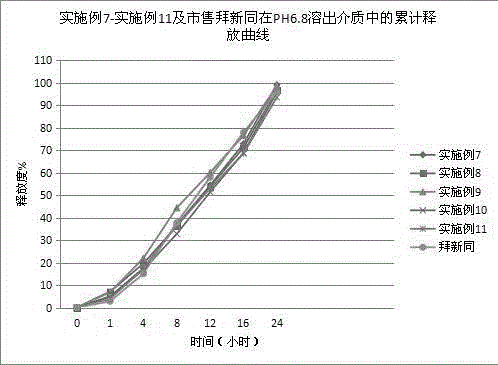
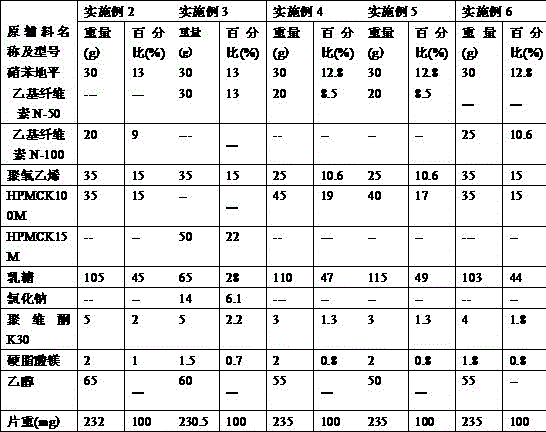
[0046] The nifedipine controlled-release tablet composition of preparation embodiment 2-11 is shown in Table 1 and Table 2,
[0047] Table 1:
[0048]
[0049] Table 2:
[0050]
[0051] Wherein, all numerical values are the dosage of nifedipine per 1000 tablets, and the unit is g, wherein the specification of example 2-example 7 is 30mg / tablet, the specification of example 8-example 9 is 20mg / tablet, and the specification of example 10-example 11 is 60mg / tablet.
[0052] In addition, commercially available Baixintong®, Jiubaoping®, and Xinran® were purchased as control drugs to compare product performance, release, and related substances.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com