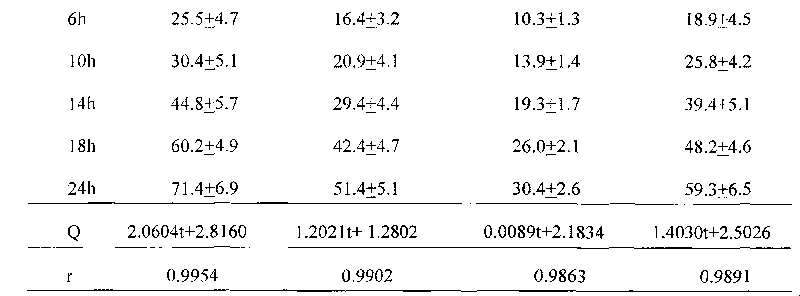Tamoxifen citrate emplastrum and preparation method thereof
A technology of tamoxifen and citric acid, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, tablet delivery, etc., which can solve the difficulty in controlling the dosage of microemulsion preparations and the failure of transdermal effects to meet the requirements , protection of personal privacy and other issues, to reduce the incidence of drug side effects, conducive to environmental protection and labor protection, and reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
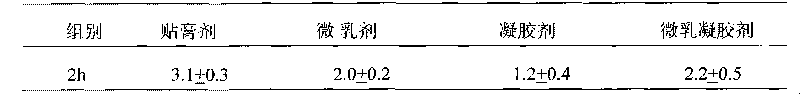
Image
Examples
Embodiment 1
[0021] Tamoxifen Citrate 1-100g
[0022] Propylene glycol 100-600g
[0023] Polysorbate 10-500g
[0024] Oleic acid 1-200g
[0025] Add pure or distilled water to 1000g
[0026] Using this prescription, tamoxifen citrate microemulsion was prepared for use. Heat the acceptable excipients to 140-160°C to melt, then lower the temperature to 90-100°C, add the adhesion enhancer and spare tamoxifen citrate microemulsion, mix well, and pour the mixture while it is still hot Put it on the release film [release paper], cover it with a layer of release film, pressurize both sides to form a film. After the above film is cooled to room temperature, the upper layer of peeling film is peeled off, and the non-woven fabric is covered on the adhesive layer and pressurized.
Embodiment 2
[0028] Tamoxifen Citrate 1-100g
[0029] Propylene glycol 100-600g
[0030] Polysorbate 10-500g
[0031] Oleic acid 1-200g
[0032] Carbomer 940 10-100g
[0033] Add pure or distilled water to 1000g
[0034] Using this prescription, tamoxifen citrate microemulsion was prepared for use. Heat the acceptable excipient ingredients to 140-160°C to melt, then lower the temperature to 90-100°C, add the adhesion enhancer and spare tamoxifen citrate microemulsion, mix well, and then put the mixture while it is hot Pour it onto the release film [release paper], cover it with a layer of release film, pressurize both sides to form a film. After the above film is cooled to room temperature, the upper layer of peeling film is peeled off, and the non-woven fabric is covered on the adhesive layer and pressurized.
Embodiment 3
[0036] Tamoxifen Citrate 1-100g
[0037] Propylene glycol 100-600g
[0038] Polysorbate 10-500g
[0039] Oleic acid 1-200g
[0040] Polyvinyl alcohol 10-100g
[0041] Add pure or distilled water to 1000g
[0042] Using this prescription, tamoxifen citrate microemulsion was prepared for use. Heat the acceptable excipient ingredients to 140-160°C to melt, then lower the temperature to 90-100°C, add the adhesion enhancer and spare tamoxifen citrate microemulsion, mix well, and then put the mixture while it is hot Pour it onto the release film [release paper], cover it with a layer of release film, pressurize both sides to form a film. After the above film is cooled to room temperature, the upper layer of peeling film is peeled off, and the non-woven fabric is covered on the adhesive layer and pressurized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com