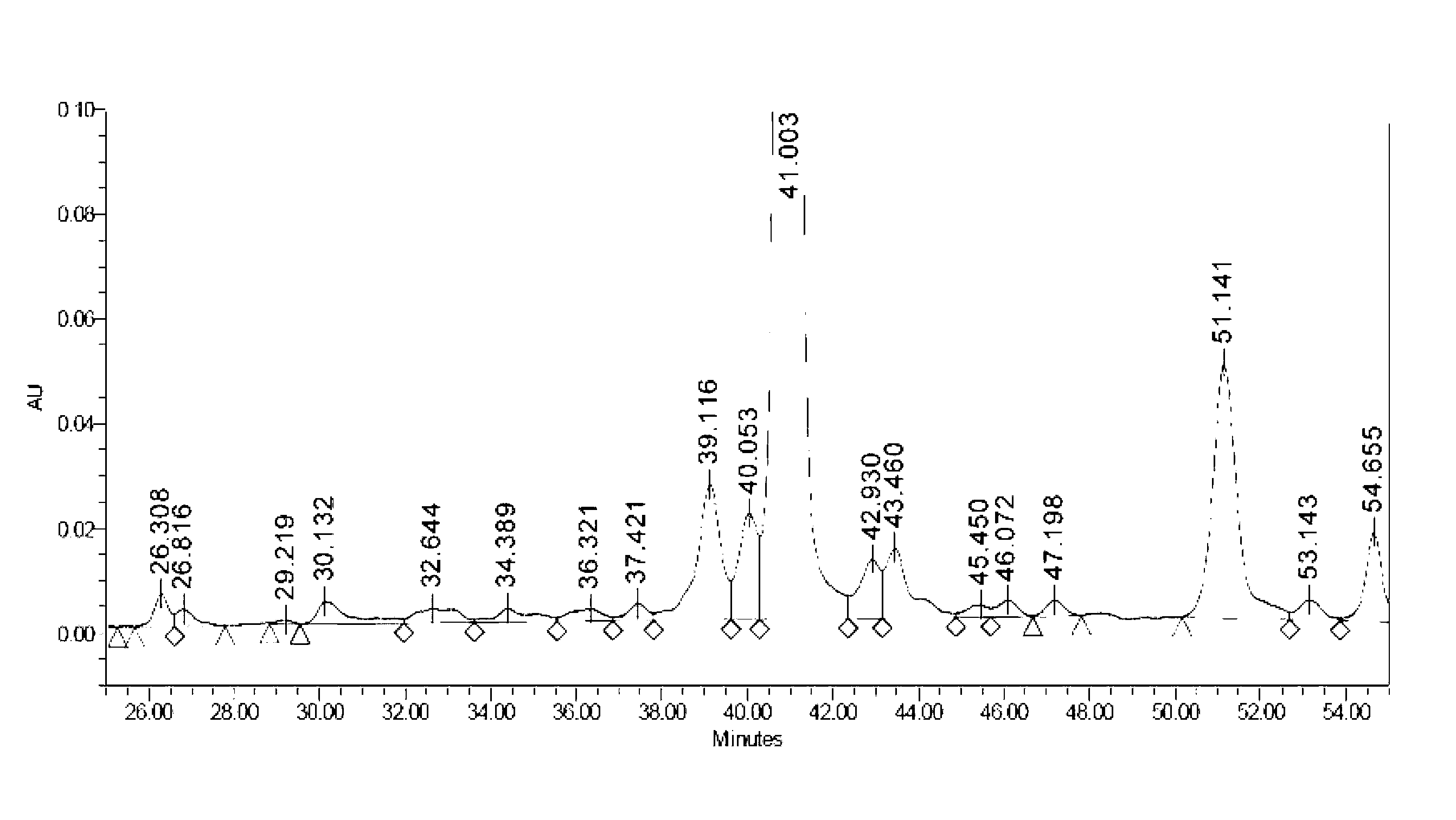
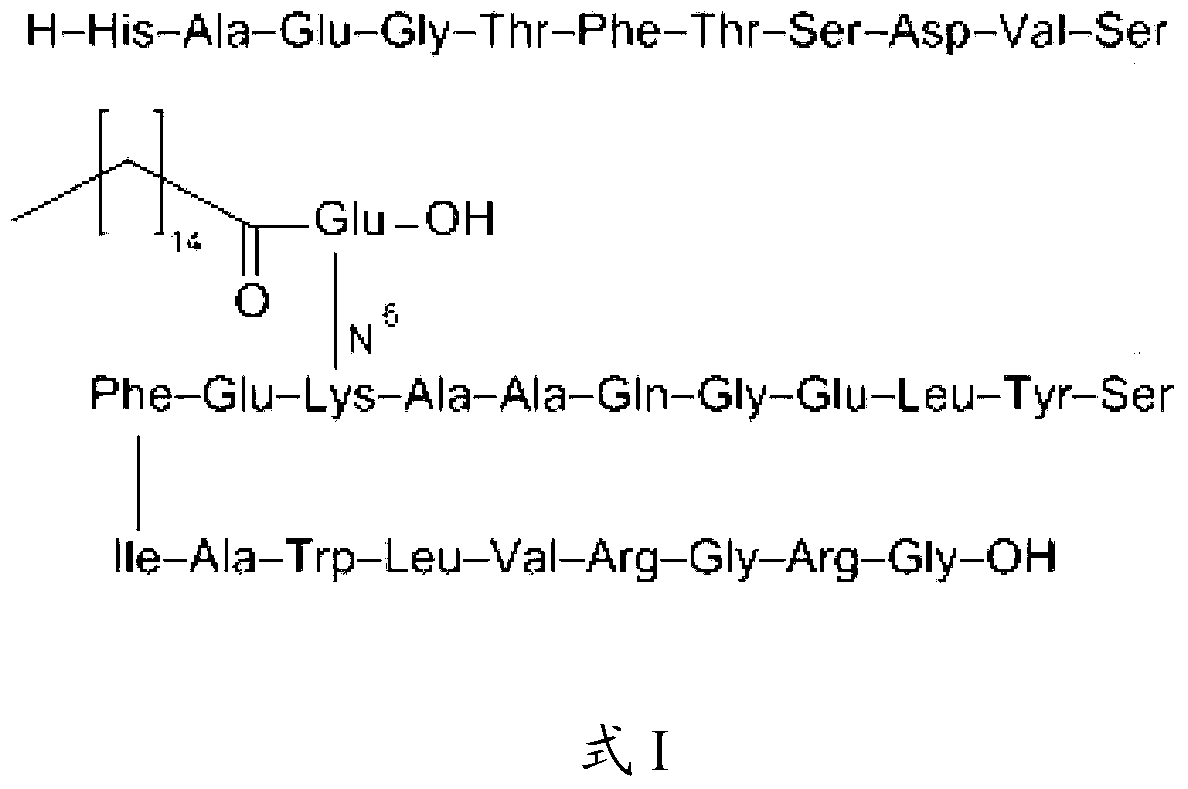Solid-phase preparation method of liraglutide
A technology for solid-phase preparation and liraglutide is applied in the field of solid-phase preparation of liraglutide, which can solve the problems of serious resin shrinkage, high technical difficulty, and many similar impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1Fmoc-Gly-Wang Resin
[0048] Weighing 100g of Wang Resin with a degree of substitution of 0.803mmol / g was added to the solid phase reaction column, washed twice with DMF, swelled with DMF for 30min, washed twice with DMF, and Fmoc-Gly-OH (35.81g, 120.5mmol) and HOBt (17.9g, 132.5mmol) was dissolved in DMF, after ice bath for 10min, DIC (16.72g, 132.5mmol) was added to activate for 3-5min, and then added to the reaction column, and DMAP (1.47g, 12.05mmol) was added at the same time, and stirred for 2h under nitrogen, Drain the reaction liquid, wash 4 times with DMF, wash 3 times with DCM, add acetic anhydride (164g, 160.6mmol) and pyridine (127g, 160.6mmol) in DCM solution to block for 8h, drain off the blocking solution, wash with DMF 6 times, and wash with DCM Twice, MeOH shrinks and dries to obtain Fmoc-Gly-Wang Resin with a substitution degree of 0.36mmol / g.
Embodiment 2
[0049] Embodiment 2 The preparation of the first polypeptide fragment-the first resin
[0050]Weigh 13.89g of Fmoc-Gly-Wang Resin with a degree of substitution of 0.36mmol / g and add it to the solid-phase reaction column, wash twice with DMF, swell with DMF for 30min, wash twice with DMF, and add Fmoc-Arg(pbf)-OH( 9.73g, 15mmol) and HOBt (2.13g, 15.8mmol) were dissolved in DMF, ice-bathed for 10min, added DIC (1.98g, 15.8mmol) to activate for 3-5min, then added to the solid-phase reaction column, stirred for 2h under nitrogen, The ninhydrin detection reaction is complete, remove the reaction solution, wash 3 times with DMF, deprotect 20% DBLK (6+6min), and wash 6 times with DMF;
[0051] Dissolve Fmoc-Gly-OH (4.46g, 15mmol) and HOBt (2.13g, 15.8mmol) in DMF, ice-bath for 10min, add DIC (1.98g, 15.8mmol) to activate for 3-5min, then add to the solid-phase reaction column During the reaction, the reaction was stirred under nitrogen for 2 hours, and the reaction was detected by n...
Embodiment 3
[0053] Example 3 The second polypeptide fragment whose sequence is shown in SEQ ID No.2 is Fmoc-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-Asp(OtBu)-Val-Ser(tBu)-Ser Preparation of (tBu)-Tyr(tBu)-Leu-Glu(OtBu)-Gly-Gln(trt)-Ala-Ala-Lys(Alloc)-Glu(OtBu)-Phe-Ile-OH
[0054] Weighing 46.5g of 2-CTC Resin with a degree of substitution of 0.86mmol / g was added to the solid-phase reaction column, washed twice with DMF, swelled with DMF for 30min, washed twice with DMF, and Fmoc-Ile-OH (42.4g, 120mmol) and DIPEA (15.5g, 120mmol) were dissolved in DMF, ice-bathed for 10min, and then added to the solid-phase reaction column. After stirring for 5 minutes under nitrogen, 15.5g of DIPEA was added again, and the reaction was continued for 1h, and 25.6g of MeOH was added to seal for 20 minutes. Dry the reaction solution, wash with DMF 6 times to obtain Fmoc-Ile-2-CTC Resin;
[0055] Add 20% DBLK to Fmoc-Ile-2-CTC Resin for deprotection (6+6min), wash 6 times with DMF, Fmoc-Phe-OH (46.5g, 120mmol), HOBt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com