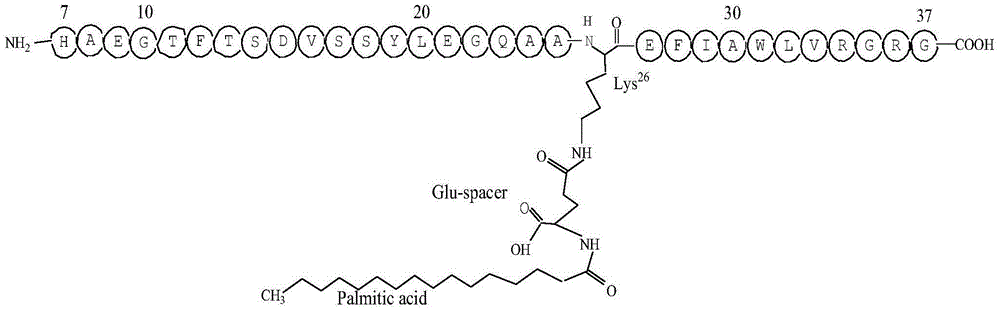
Solid-liquid combined preparation method for liraglutide
A technology of liraglutide and solution, applied in the field of polypeptide drug preparation, can solve the problems of unsuitable industrial-scale production, found that the purity and yield are not high, and achieves the advantages of being beneficial to large-scale industrial production, having good water solubility, and solving the problem of excessive peptide impurities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of Fmoc-Lys(N-ε-(γ-Glu(N-α-Boc)-OtBu)-OH
[0031] Accurately weigh Fmoc-Lys-OH183.8g (0.5mol) and sodium carbonate 63.6 (0.6mol) and dissolve them in 1200mL of water, slowly add Boc-Glu(OSu)-OtBu tetrahydrofuran solution (200.3 g, 0.5mol) / 1000ml, stir the reaction, TLC monitors the reaction end point, after the reaction is complete, spin off the THF, add 10% citric acid aqueous solution under the ice water bath to adjust the pH value of the solution to 2~3, and extract 3 times with 1000ml ethyl acetate , the organic phases were combined, washed 3 times with 200ml saturated brine, dried over anhydrous sodium sulfate, concentrated by rotary evaporation to 1000ml, and left to stand for crystallization to obtain Fmoc-Lys(N-ε-(γ-Glu(N-α-Boc)- OtBu)-OH 253.6g, yield 73.5%.
Embodiment 2
[0032] Embodiment 2: Synthesis of Fmoc-Gly-WangResins
[0033] Put the carrier Wang resin 400.0g (sub=0.47mmol / g) in the synthesis column, wash twice with 2400mL DMF, add 4000mL DCM to swell for 30min; after filtering off the DCM, add the mixed DCM of Fmoc-Gly-OH / DIC / HOBT Solution [Weigh 118.8g (400mmol) Fmoc-Gly-OH and 64.8g (480mmol) HOBT into the glycine activation bottle, add 2000mL of DMF and DCM mixed solution with a volume ratio of 1:1 and stir to dissolve. Add 76.4ml (480mmol) DIC, activate for 5 minutes], add 4.8g (4mmol) DMAP after 10min of reaction; react for 3h, remove the reaction solution, wash twice with 4000mL DMF, add 2400mL of capping reagent (480ml acetic anhydride and 408ml pyridine Dissolved in 1512mL DMF) reacted for 2h, filtered off the reaction solution, washed twice with DMF, DCM, methanol, and vacuum dried to obtain 436.6g of Fmoc-Gly-WangResins ; The degree of substitution was measured by sampling to be 0.30mmol / g.
Embodiment 3
[0034] Example 3: Synthesis of Fmoc-Gly-CTCResins
[0035] Weigh 50.0g (sub=0.40mmol / g) of CTC resin and place it in a synthesis column, wash it twice with 240mL DMF, add 240mL DCM to swell for 30min; DCM / DMF (3 / 1, volume ratio) solution 150ml, add DIPEA6.6ml (40mmol) after stirring, drum N 2 React for 60min, remove the reaction liquid, add DCM / CH 3 300ml of OH / DIPEA (volume ratio 17:2:1) mixed solution was capped 3 times for 10min each time; then washed twice with DMF, DCM and methanol respectively, and dried in vacuum to obtain 53.80g of Fmoc-Gly-CTCResins. The measured degree of substitution is 0.29mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com