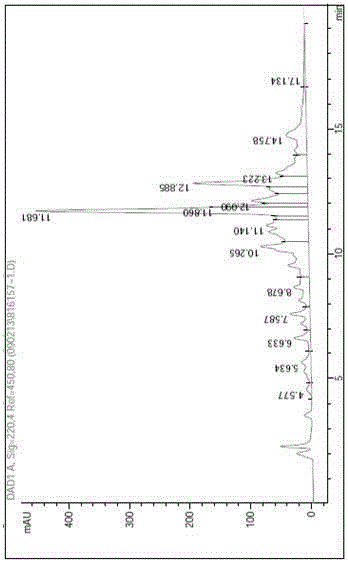
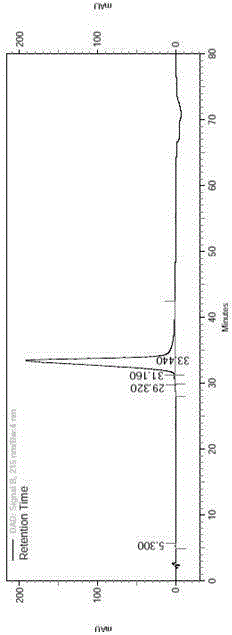
Preparation method for liraglutide
A technology of liraglutide and a synthesis method, which is applied in the field of chemical synthesis of the main chain of liraglutide, can solve the problems of low reaction yield, numerous side reactions of polypeptide chain and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1 Preparation of Liraglutide.
[0110] Weigh Fmoc-Gly 37 -WangResin (0.35mmol / g, 12.5g) was placed in the reactor, and DMF-swelled resin was added to a bed twice the volume of the resin, stirred with air blowing for 2.0 hours, and the DMF was drained. Add 20% piperidine / DMF, pump nitrogen for 30 minutes, then drain, wash with DMF 5 times, 1 minute each time, drain DMF for use, add the activated reaction solution [Fmoc-Arg(pbf)-OH (8.5g, 3eq), HOBt (1.77g, 3eq.) in a beaker, add appropriate amount of DMF to dissolve, ice bath, stir until completely dissolved, 10 minutes before the reaction, add DIC (1.65g, 3eq.) to activate , the temperature of the solution was controlled at 10°C], and the reaction was stirred and reacted at 30°C for 3 hours with nitrogen blowing. After the ninhydrin test was negative, the reaction solution was drained and washed with DMF. Use 20% piperidine / DMF for deprotection treatment, that is, remove the Fmoc group. After 30 minutes, use ...
Embodiment 2
[0116] Embodiment 2 Arg 34 Preparation of GLP-1.
[0117] Weigh Fmoc-Gly 37 -WangResin (0.35mmol / g, 12.5g) was placed in the reactor, and DMF swelling resin was added to a bed twice the resin volume, stirred for 2.0-4.0 hours with air blowing, and the DMF was drained. Add 20% piperidine / DMF, pump nitrogen for 30 minutes, then drain, wash with DMF 5 times, 1 minute each time, drain DMF for use, add the activated reaction solution [Fmoc-Arg(pbf)-OH (8.5g, 3eq), HOBt (1.77g, 3eq.) in a beaker, add appropriate amount of DMF to dissolve, ice bath, stir until completely dissolved, 10 minutes before the reaction, add DIC (1.65g, 3eq.) to activate , The temperature of the solution is controlled at 10°C], and the reaction is stirred and reacted at 30°C for 2.5-3.5 hours with nitrogen blowing. After the ninhydrin test was negative, the reaction solution was drained and washed with DMF. Use 20% piperidine / DMF for deprotection treatment, that is, remove the Fmoc group. After 30 minute...
Embodiment 3
[0123] Embodiment 3 Arg 34 Preparation of GLP-1.
[0124] Weigh Fmoc-Gly 37 -WangResin (0.35mmol / g, 12.5g) was placed in the reactor, and DMF swelling resin was added to a bed twice the resin volume, stirred for 2.0-4.0 hours with air blowing, and the DMF was drained. Add 20% piperidine / DMF, pump nitrogen for 30 minutes, then drain, wash with DMF 5 times, 1 minute each time, drain DMF for use, add the activated reaction solution [Fmoc-Arg(pbf)-OH (8.5g, 3eq), HOBt (1.77g, 3eq.) in a beaker, add appropriate amount of DMF to dissolve, ice bath, stir until completely dissolved, 10 minutes before the reaction, add DIC (1.65g, 3eq.) to activate , the temperature of the solution was controlled at 10°C], and the reaction was stirred under nitrogen blowing at 30°C for 2.5-3.5 hours. After the ninhydrin test was negative, the reaction solution was drained and washed with DMF. Use 20% piperidine / DMF for deprotection treatment, that is, remove the Fmoc group. After 30 minutes, use DM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com