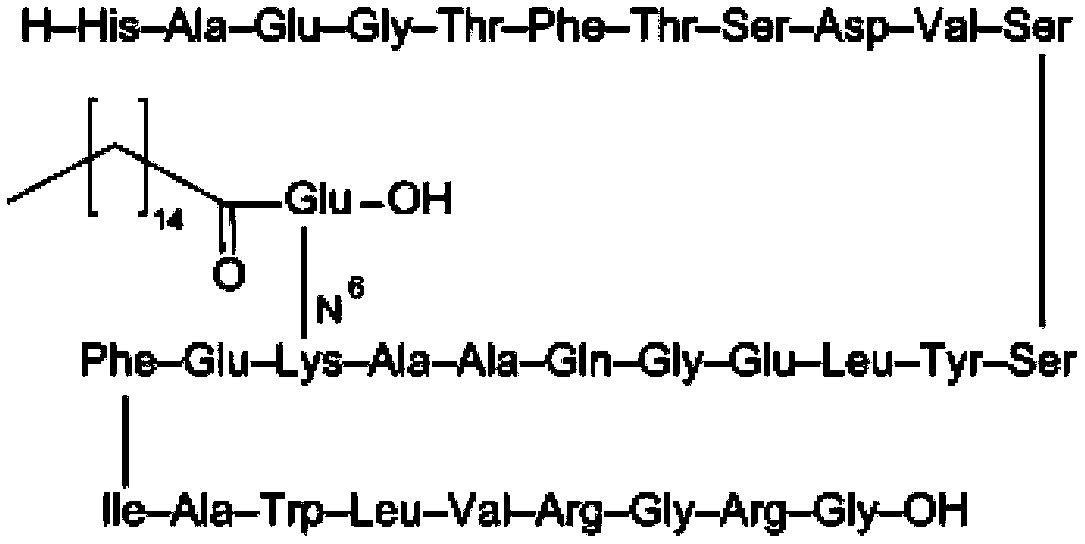Synthetic method of liraglutide
A synthesis method and technology of liraglutide, applied in the field of synthesis of liraglutide, can solve the problems of incomplete reaction, unfavorable separation and purification, high cost, improve purity and yield, facilitate purification operation, and reduce impurities the effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
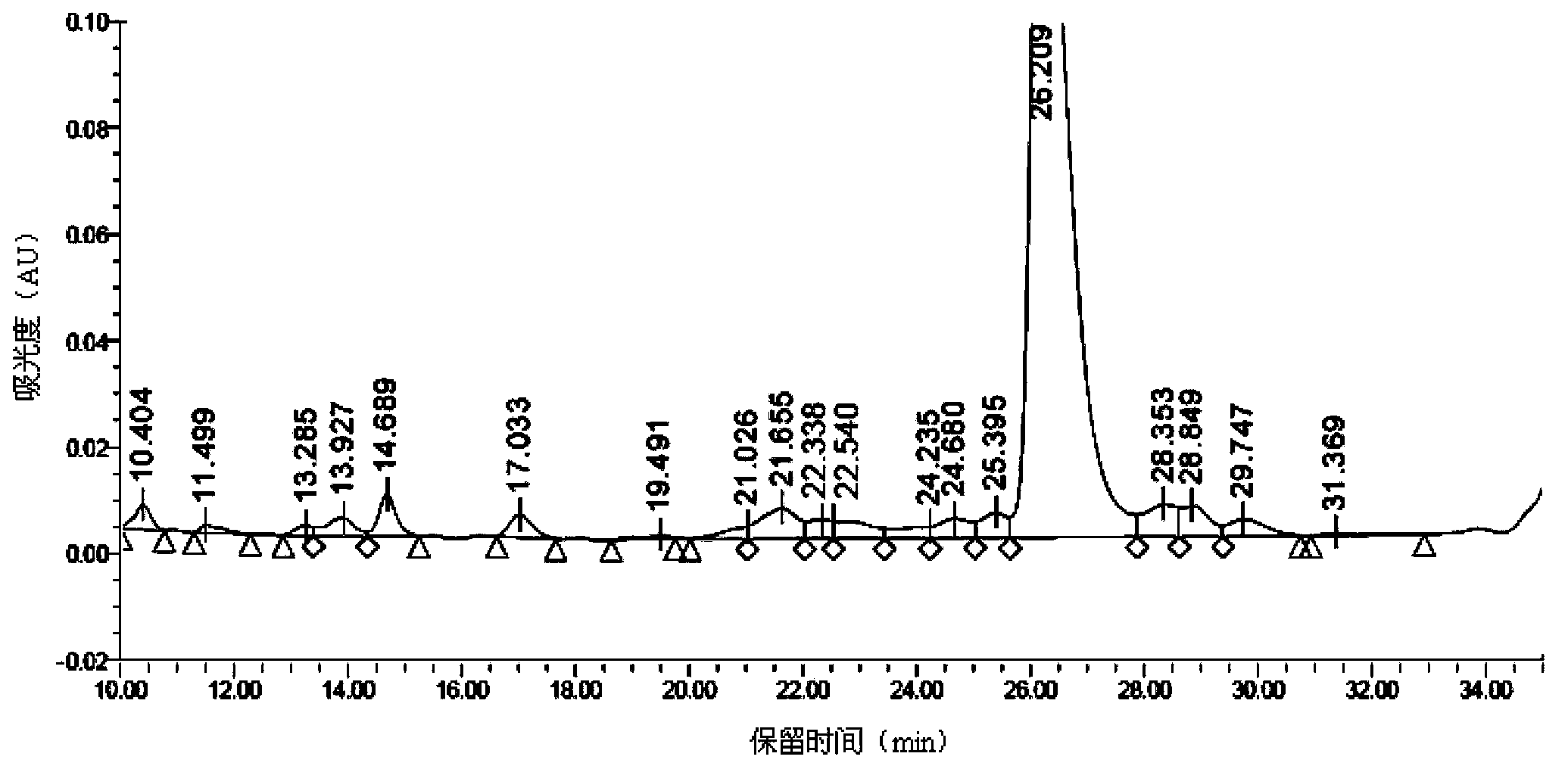
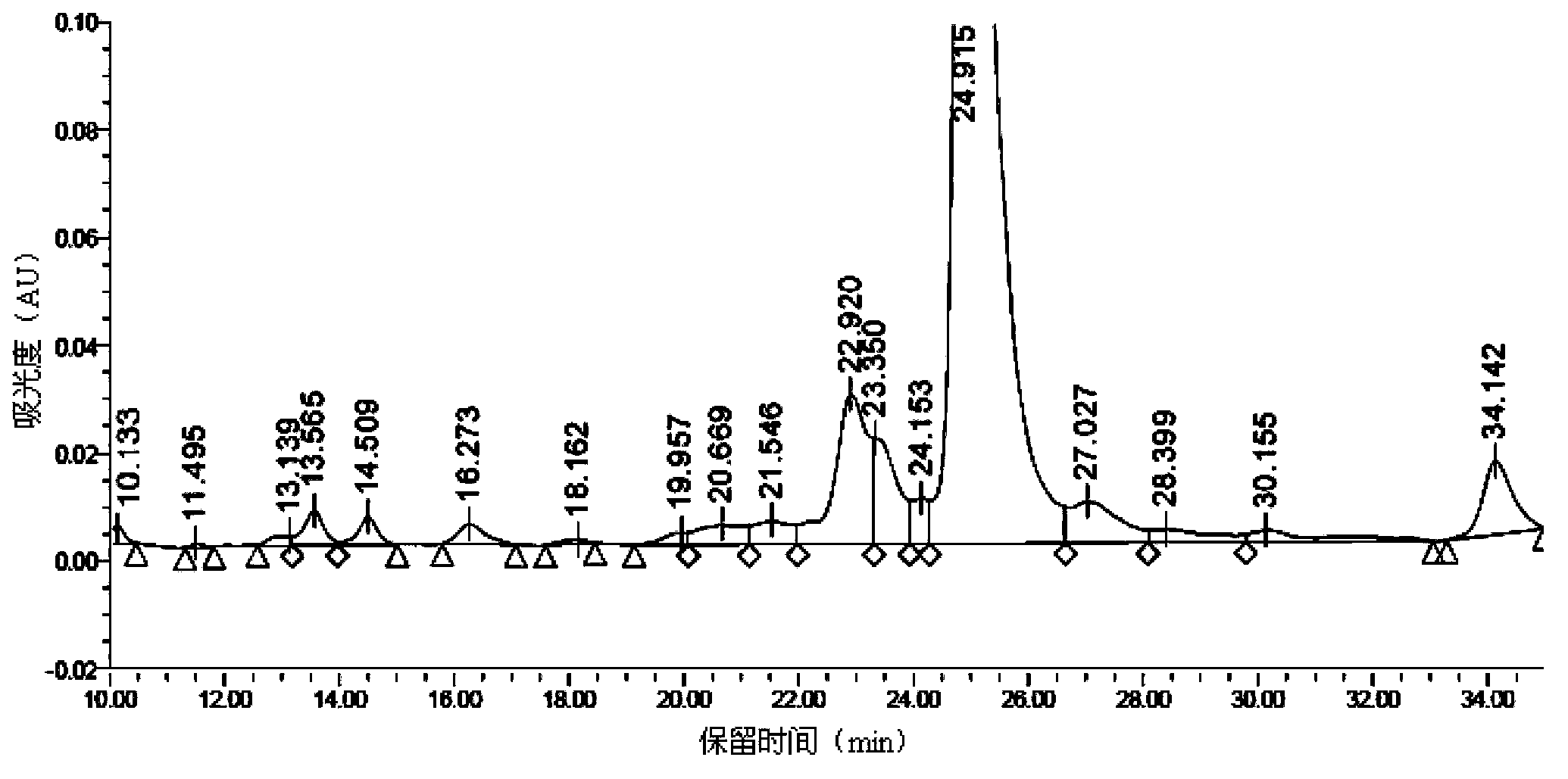
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of the first polypeptide fragment
[0041] Weigh 40g of 2-CTC resin with a substitution degree of 0.5mmol / g and add it to the solid phase reaction column, wash with DMF twice, swell with DMF for 30min, wash with DMF twice, add Fmoc-Thr(tBu)-OH (31.80g, 80mmol) and DIPEA (14ml, 80mmol) were dissolved in DMF, ice-bathed for 10min, and then added to the above solid-phase reaction column, stirred and reacted with nitrogen for 5 minutes, then added 14ml DIPEA again, continued to react for 1h, and added 12.8g MeOH to seal for 20 minutes, The reaction solution was drained and washed 6 times with DMF to obtain Fmoc-Thr(tBu)-2-CTC resin.
[0042] Add 20% DBLK to Fmoc-Thr(OtBu)-2-CTC resin for deprotection (6+6min), wash with DMF 6 times, Fmoc-Phe-OH (30.99g, 80mmol), HOBt (11.35g, 84mmol) Dissolve PyBOP (41.63g, 80mmol) in DMF, ice bath for 10 minutes, add 27.9ml DIPEA to activate for 5 minutes, then add to the above solid-phase reaction column, stir and r...
Embodiment 2
[0046] Example 2 Preparation of the second polypeptide fragment
[0047] Weigh 40g of 2-CTC resin with a substitution degree of 0.5mmol / g and add it to the solid phase reaction column, wash it twice with DMF, swell with DMF for 30min, wash twice with DMF, add Fmoc-Glu(OtBu)-OH (35.04g, 80mmol) and DIPEA (14ml, 80mmol) were dissolved in DMF, ice-bathed for 10min, and then added to the above solid-phase reaction column, stirred and reacted with nitrogen for 5 minutes, then added 14ml DIPEA again, continued to react for 1h, and added 12.8g MeOH to seal for 20 minutes, The reaction solution was drained and washed 6 times with DMF to obtain Fmoc-Glu(OtBu)-2-CTC resin.
[0048] Add 20% DBLK to Fmoc-Glu(OtBu)-2-CTC resin for deprotection (6+6min), wash with DMF 6 times, Fmoc-Leu-OH (28.28g, 80mmol), HOBt (11.35g, 84mmol) Dissolve PyBOP (41.63g, 80mmol) in DMF, ice bath for 10 minutes, add 27.9ml DIPEA to activate for 5 minutes, then add to the above solid-phase reaction column, stir...
Embodiment 3
[0052] Example 3 Preparation of the third polypeptide fragment
[0053] Weigh 40g of 2-CTC resin with a substitution degree of 0.5mmol / g and add it to the solid phase reaction column, wash it twice with DMF, swell with DMF for 30min, wash twice with DMF, add Fmoc-Glu(OtBu)-OH (35.04g, 80mmol) and DIPEA (14ml, 80mmol) were dissolved in DMF, ice-bathed for 10min, and then added to the above solid-phase reaction column, stirred and reacted with nitrogen for 5 minutes, then added 14ml DIPEA again, continued to react for 1h, and added 12.8g MeOH to seal for 20 minutes, The reaction solution was drained and washed 6 times with DMF to obtain Fmoc-Glu(OtBu)-2-CTC resin.
[0054] Add 20% DBLK to Fmoc-Glu(OtBu)-2-CTC resin for deprotection (6+6min), wash with DMF 6 times, Fmoc-Lys(Alloc)-OH (28.28g, 80mmol), HOBt (11.35g , 84mmol) and PyBOP (41.63g, 80mmol) were dissolved in DMF, ice-bathed for 10 minutes, added 27.9ml DIPEA to activate for 5 minutes, then added to the above solid-phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com