Synthetic method for liraglutide
A technology of liraglutide and a synthesis method, which is applied in the field of peptide synthesis and preparation, can solve the problems of difficult removal of impurities, long synthesis cycle, high cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
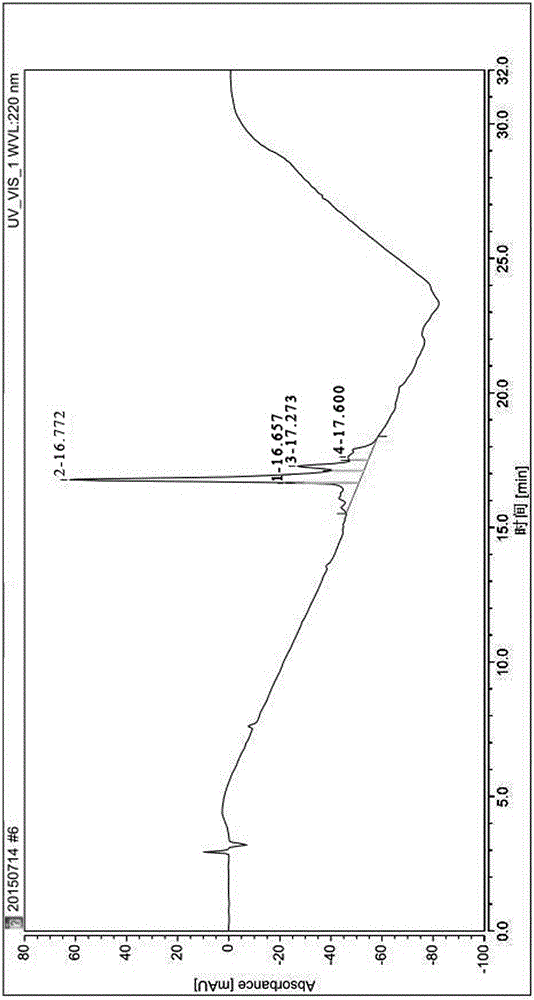
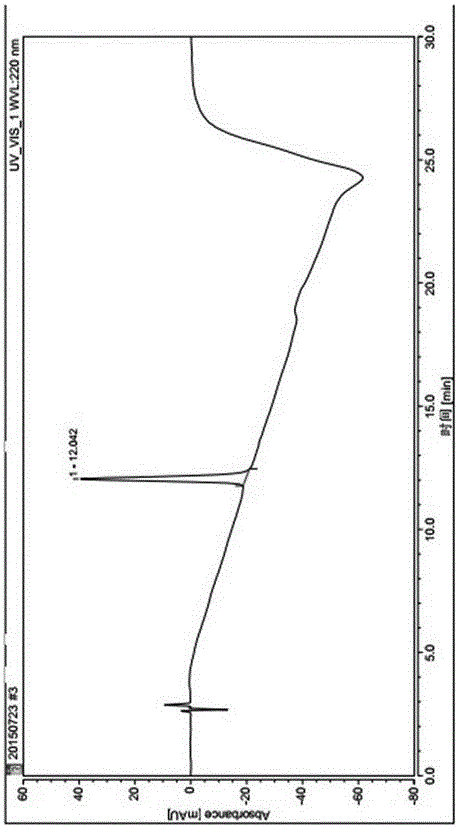
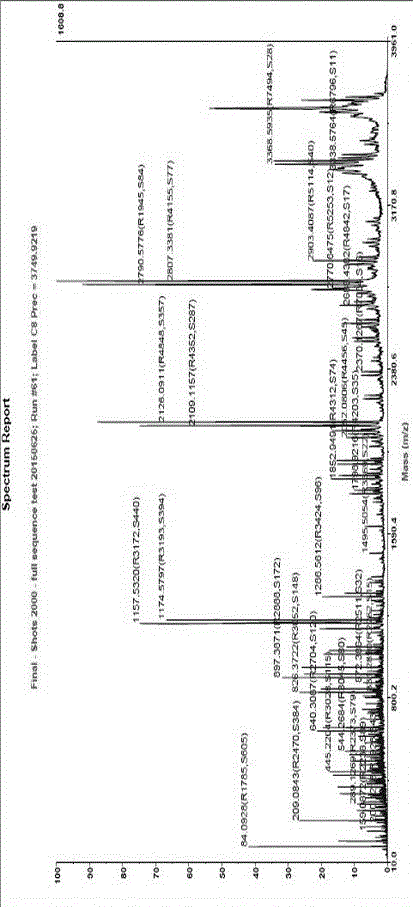
Image
Examples
Embodiment 1
[0063] Embodiment 1, a synthetic method of liraglutide, comprises the following steps:
[0064] A. Fragment condensation into various liraglutide intermediates:
[0065] (1) Synthesis of R-His(R1)-Ala-Glu(R2)-Gly-R3;
[0066] (2) Synthesis of R-Thr(R4)-Phe-Thr(R4)-Ser(R4)-Asp(R5)-R3;
[0067] (3) Synthesis of R-Val-Ser(R4)-Ser(R4)-Tyr(R4)-Leu-Glu(R2)-Gly-R3;
[0068] (4) Synthesis of R-Gln-Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu(R2)]-Lys-Glu(R2)-Phe-R3;
[0069] (5) Synthesis of R-Ile-Ala-Trp(R6)-Leu-Val-Arg(R7)-Gly-Arg(R7)-Gly-R3;
[0070] (6) Synthesis of R-His(R1)-Ala-Glu(R2)-Gly-Thr(R4)-Phe-Thr(R4)-Ser(R4)-Asp(R5)-R3;
[0071] (7) R-His(R1)-Ala-Glu(R2)-Gly-Thr(R4)-Phe-Thr(R4)-Ser(R4)-Asp(R5)-Val-Ser(R4)-Ser( Synthesis of R4)-Tyr(R4)-Leu-Glu(R2)-Gly-R3;
[0072] (8) R-His(R1)-Ala-Glu(R2)-Gly-Thr(R4)-Phe-Thr(R4)-Ser(R4)-Asp(R5)-Val-Ser(R4)-Ser( R4)-Tyr(R4)-Leu-Glu(R2)-Gly-Gln-Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu(R2)]-Lys-Glu(R2)-Phe-R3 Synthesis;
[0073] (9) R-Thr(R4)-Phe...
Embodiment 2
[0098] Example 2, in the synthesis method of liraglutide described in Example 1: the coupling system used in the synthesis process of the 14 polypeptide fragments is selected from: single condensing agent: DIC, DCC, EDC , chloroacetyl chloride, azide, TBTU, PyBOP, HBTU; or two-system condensing agent: DIC / HOBT, DCC / HOBT, EDC / HOBT.
Embodiment 3
[0099] Example 3, in a synthetic method of liraglutide described in Example 1: (1) R-Gln-Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu]-Lys-Glu( The synthesis of R2)-Phe-R3 adopts pure liquid phase synthesis, including a variety of fragment synthesis methods, the specific fragments are as follows:
[0100] R-Gln-Ala-OH, R-Gln-Ala-Ala-OH, R-Gln-Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu]-Lys(R8)-OH, R-Gln -Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu]-Lys(R8)-Glu(R2)-R3, R-Phe-R3, R-Glu(R2)-Phe-R3, R- N6-[N-(1-oxohexadecyl)-Glu]-Lys(R8)-Glu(R2)-Phe-R3, R-Ala-N6-[N-(1-oxohexadecyl)-Glu]-Lys(R8) -Glu(R2)-Phe-R3, R-Ala-Ala-N6-[N-(1-oxohexadecyl)-Glu]-Lys(R8)-Glu(R2)-R3;
[0101] (2) Synthesis of N-(1-oxohexadecyl)-Glu(R2)]-R3:
[0102] The target product is obtained by reacting NH2-Glu(R2)-OH with active ester of stearic acid; the single condensing agent and condensing agent compound used in the synthesis of active ester method are: DIC, DCC, EDC, TBTU, PyBOP, HBTU , DIC / HOBT, DCC / HOBT, EDC / HOBT; or use N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com