Method for synthesizing liraglutide
A technology of liraglutide and a synthesis method, which is applied in the field of polypeptide drug synthesis, can solve the problems of low purity and yield, complicated operation steps, high cost and the like, and achieves high purity and yield, simple operation steps and few impurities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
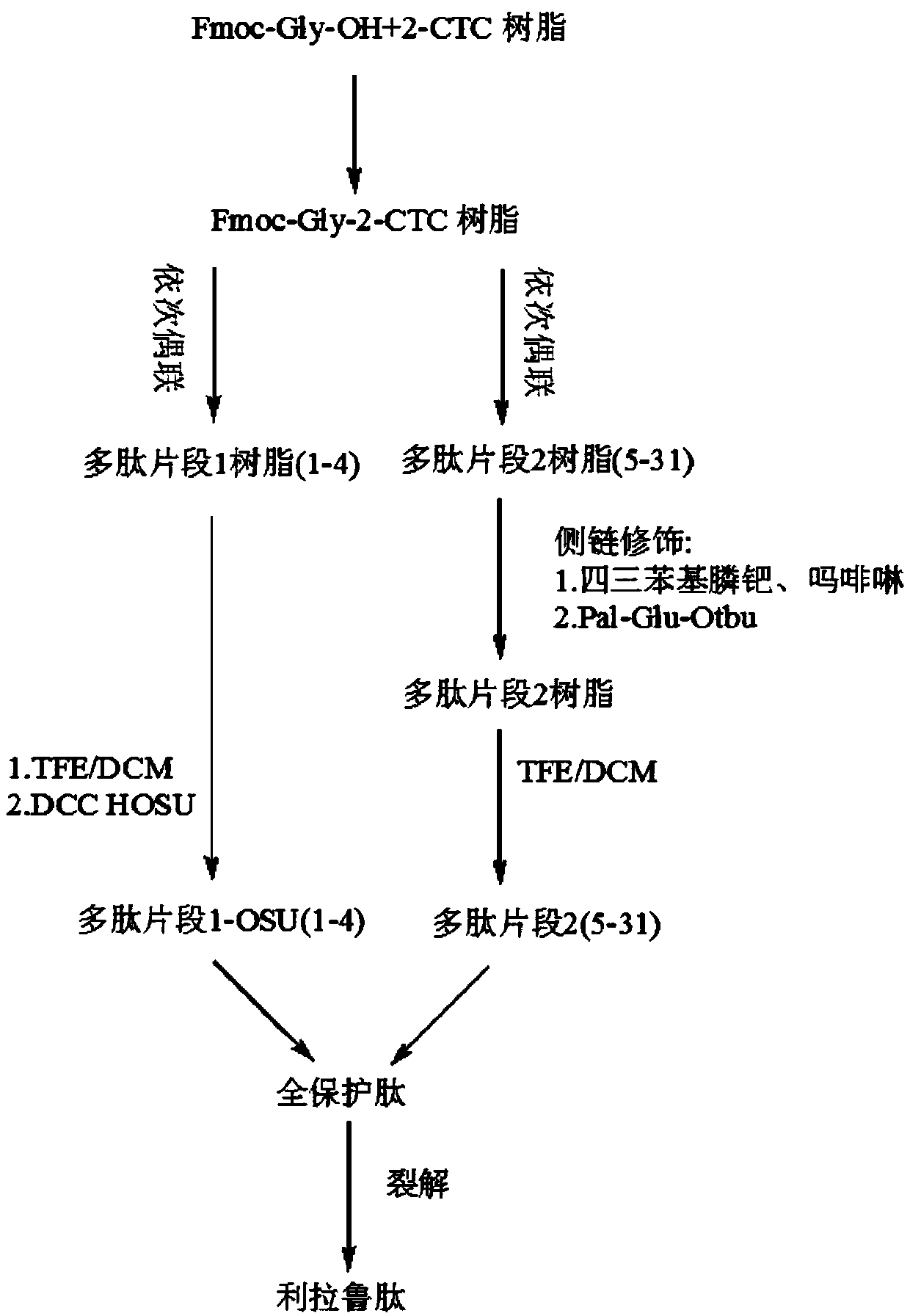
[0045] Embodiment 1, a synthetic method of liraglutide, the steps are as follows:
[0046] (1) Solid-phase synthesis of the 1-4 amino acids of the liraglutide sequence as the first fragment,
[0047] (2) Solid-phase synthesis of amino acids 5-31 of the liraglutide sequence, removing the side chain protecting group of Lys at position 20 and coupling Pal-γ-Glu-Otbu as the second fragment;
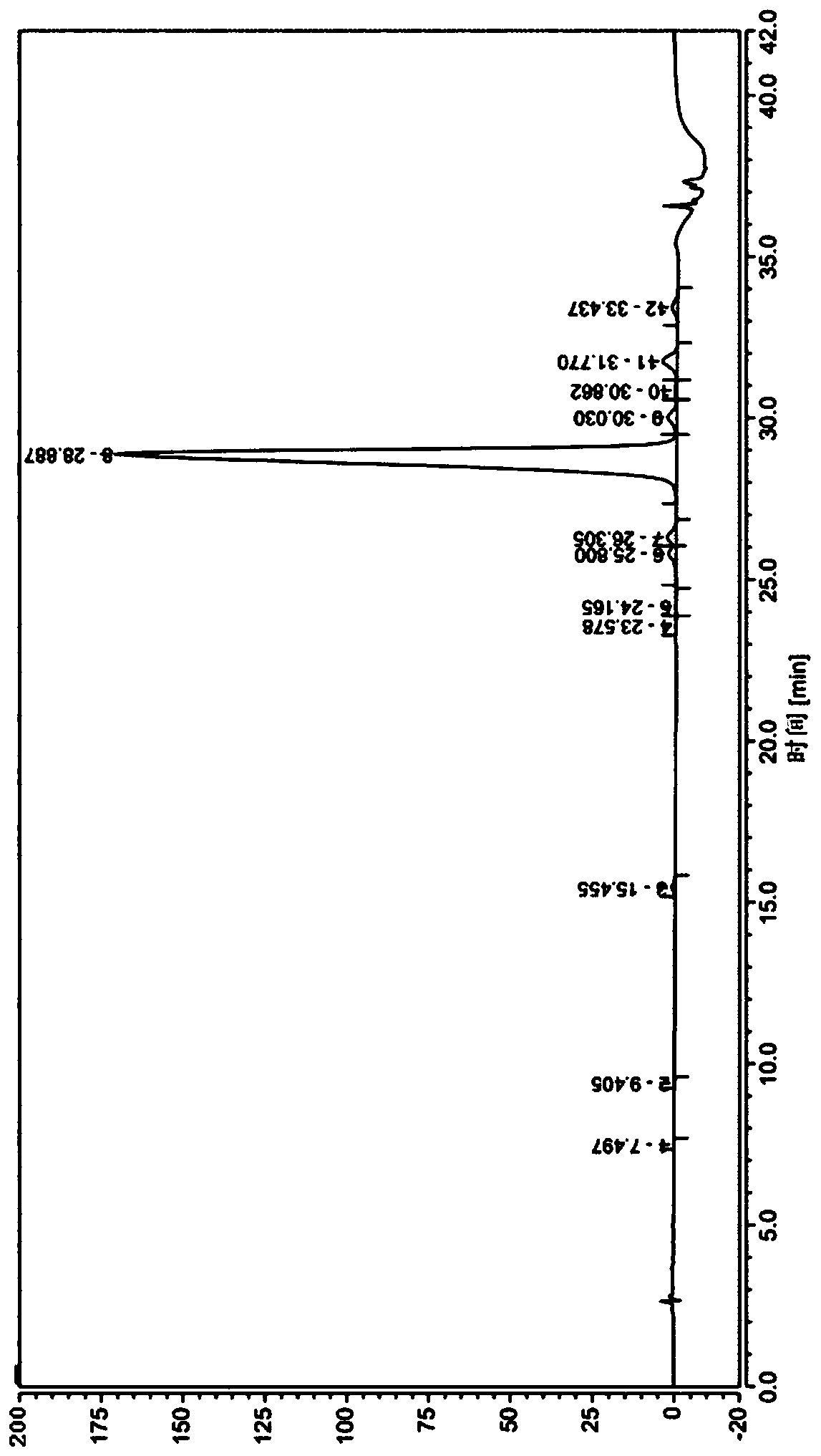
[0048] (3) The first fragment is coupled with the second fragment in liquid phase to synthesize fully protected liraglutide, which is cleaved and precipitated to obtain liraglutide.
Embodiment 2
[0049] Example 2, in the synthesis method of liraglutide described in Example 1: in step (1), the resin used for the first fragment and the second fragment is 2-CTC resin. In step (2), the side chain protecting group of Lys at position 20 in the second fragment is selected as Alloc removed by catalytic hydrogenation. In step (1) and / or (2), the condensing agent used to synthesize the first fragment and the second fragment is one or more of DIC / HOBt, HBTU / HOBT / DIEA, PyBop / HOBT / DIEA; The reaction solvent is one or more combinations of DCM, DMF, NMP, and DMSO; the Fmoc removal reagent used is v / v 15% piperidine / DMF solution. In step (1) and / or (2), the fully protected lysis reagent of the first fragment and the second fragment is v / v15%%TFE / DCM or v / v0.05%TFA / DCM. The condensing agent used in the liquid phase coupling of the first fragment and the second fragment in step (3) is a combination of DCC / HOSu or EDC·HCl / HOSU. The lysis reagent used in step (3) is TFA:Thioanisole:Anis...
Embodiment 3
[0050] Example 3, in the synthesis method of liraglutide described in Example 1: in step (1), the resin used for the first fragment and the second fragment is 2-CTC resin. In step (2), the side chain protecting group of Lys at position 20 in the second fragment is selected as Alloc removed by catalytic hydrogenation. In step (1) and / or (2), the condensing agent used to synthesize the first fragment and the second fragment is one or more of DIC / HOBt, HBTU / HOBT / DIEA, PyBop / HOBT / DIEA; The reaction solvent is one or more combinations of DCM, DMF, NMP, and DMSO; the Fmoc removal reagent used is v / v 25% piperidine / DMF solution. In step (1) and / or (2), the fully protected lysis reagent of the first fragment and the second fragment is v / v 25% TFE / DCM or v / v 0.15% TFA / DCM. The condensing agent used in the liquid phase coupling of the first fragment and the second fragment in step (3) is a combination of DCC / HOSu or EDC·HCl / HOSU. The lysis reagent used in step (3) is TFA:Thioanisole:A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com