Liraglutide sustained-release microsphere preparation and preparation method thereof
A slow-release microsphere preparation, liraglutide technology, applied in the direction of pharmaceutical formulations, peptide/protein components, medical preparations of non-active ingredients, etc., can solve the problem of sudden release, unsatisfactory encapsulation rate and drug loading, Poor patient compliance, frequent dosing times and other issues, to achieve the effect of sustained slow release, high drug loading, and no obvious burst release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of liraglutide sustained-release microsphere preparation by S / 0 / W double emulsification-solvent evaporation method
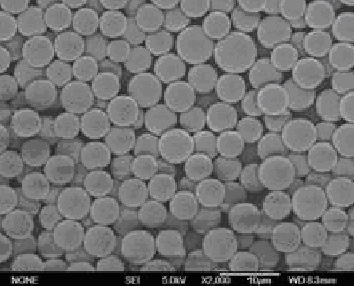
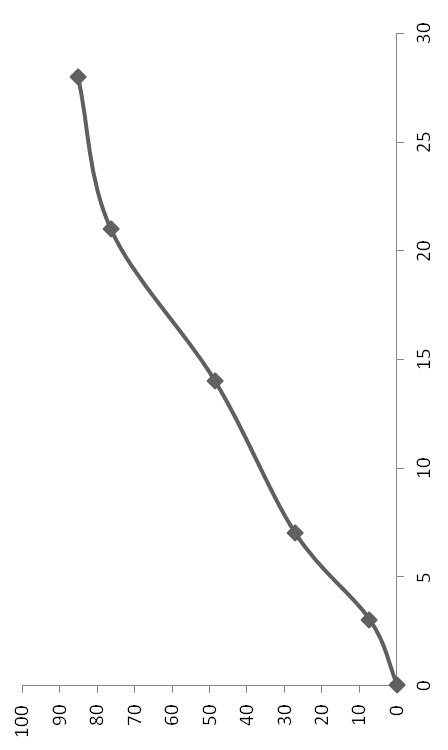
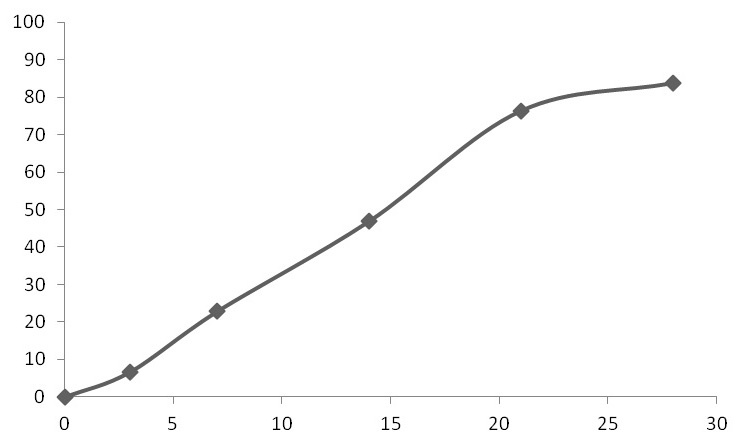
[0049] PLGA-PCL (polylactic acid: glycolic acid ratio is 60:40, Mw=10,000, polylactic acid-glycolic acid copolymer: polycaprolactone ratio is 1:2) 200mg dissolved in 4.0ml organic solvent (dichloro The ratio of methane:ethyl acetate is 2:1) to make the oil phase, disperse 20mg of liraglutide into the colostrum of the oil phase, disperse to form S / 0, put 50m1 of the solution containing 2%PVA in a stirring container , quickly add colostrum into the external water phase under high-speed stirring (5,000rpm) and fully homogenize. After three minutes, reduce the speed to 300rpm, add 0.75% polyvinyl alcohol solution, stir at room temperature and under reduced pressure for 6 hours, and the microspheres After hardening, centrifuge and wash, then freeze-dry. The encapsulation efficiency of liraglutide microspheres was 87%, and the particle si...
Embodiment 2
[0050] Example 2 S / O / W 2 Preparation of Liraglutide Sustained-release Microspheres by Double Emulsification-Solvent Evaporation Method
[0051] PLGA-PCL (the ratio of polylactic acid: glycolic acid is 50:50, Mw=15,000, the ratio of polylactic acid-glycolic acid copolymer: polycaprolactone is 1:4) 250mg dissolved in 2.5m1 methylene chloride For the oil phase, add 50 mg of liraglutide to the above oil phase, disperse to form S / O colostrum, put 50 ml of a solution containing 1% polyvinyl alcohol (PVA) in a stirring container, and stir the colostrum at high speed ( 10,000rpm) and quickly added to the external water phase to fully homogenize. After one minute, reduce the speed to 600rpm, add 0.5% polyvinyl alcohol solution, stir at room temperature and reduced pressure for 6 hours, centrifuge and wash the microspheres after hardening. Just freeze dry. The encapsulation efficiency of liraglutide microspheres was 92%, and the particle size was <80um.
Embodiment 3
[0052] Example 3 Preparation of Liraglutide Sustained Release Microspheres by Spray Drying
[0053] Dissolve 100mg of PLGA (the ratio of polylactic acid:glycolic acid is 75:25, Mw=10,000) in 5.0ml of organic solvent (the ratio of dichloromethane:methanol is 3:1). Disperse 20 mg of liraglutide and 2.0 mg of trehalose into 2.0 ml of water for injection, and vortex to dissolve the drug completely. Add the liraglutide solution into the organic phase, stir at 15,000 rpm for three minutes at high speed to form a W / 0 emulsion, spray it into the low-temperature ethanol covered with liquid nitrogen through a spray dryer in an atomized state; the ethanol volatilizes The dichloromethane in the PLGA droplets was extracted; the liquid nitrogen was evaporated under freezing conditions, and the microspheres were obtained by freeze-drying. The encapsulation efficiency of liraglutide microspheres was 85%, and the particle size was <80um.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com